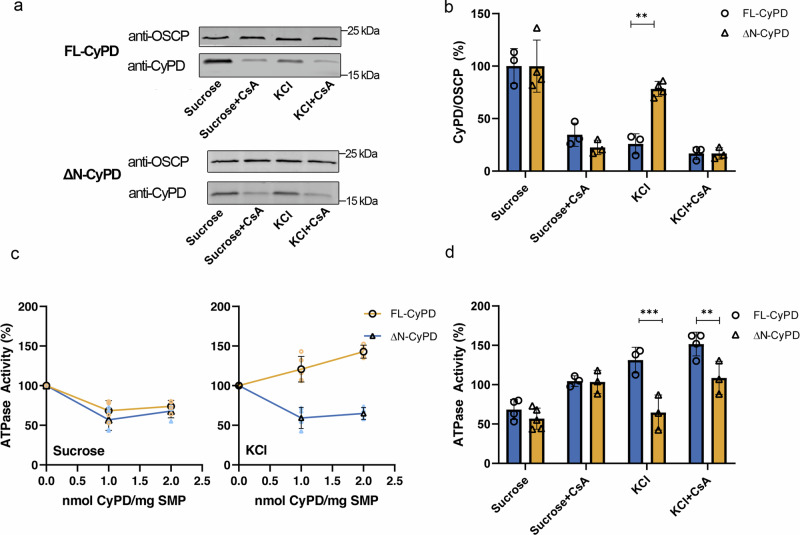
Fig. 4. Binding and modulation of F-ATP synthase by FL-CyPD and ΔN-CyPD.
a Pig heart SMP depleted of endogenous CyPD (1 mg/ml) were incubated at 25°C for 15 min with 1 nmol of either FL-CyPD or ΔN-CyPD/mg SMP under two different conditions, namely in the sucrose-based buffer (250 mM sucrose) or the KCl-based buffer (125 mM KCl) supplemented with 10 mM KH2PO4, in the presence or absence of 2 μM CsA. Immunoprecipitation of OSCP was performed, followed by Western blotting with anti-OSCP or anti-CyPD antibodies (representative experiment out of three). b Each immunodetected band was analyzed by densitometry, and the ratio between the peak area of CyPD (FL-CyPD or ΔN-CyPD) and that of the corresponding OSCP subunit was measured and expressed relative to the ratio obtained in the sucrose buffer in the absence of CsA, which was taken as 100% (values are mean ± S.D. of at least three independent experiments). c ATPase activity of pig heart SMP depleted of endogenous CyPD and exposed to different concentrations of either FL-CyPD (◯) or ΔN-CyPD (△) in the sucrose-based buffer (left panel) or the KCl-based buffer (right panel). The oligomycin-sensitive ATP hydrolysis rate was determined spectrophotometrically. Mean ± S.D. values of at least four independent experiments are shown in black. d ATPase activity of pig heart SMP exposed to 1 nmol/mg SMP of either FL-CyPD or ΔN-CyPD in either sucrose-based buffer or KCl-based buffer in the presence or absence of 2 μM CsA. The ATPase activity in the absence of FL-CyPD or ΔN-CyPD was taken as 100%, and reported values are mean ± S.D. of at least 3 independent experiments. Data were analysed according to the two-way ANOVA analysis followed by Bonferroni’s multiple comparisons test (**p < 0.01; ***p < 0.005).