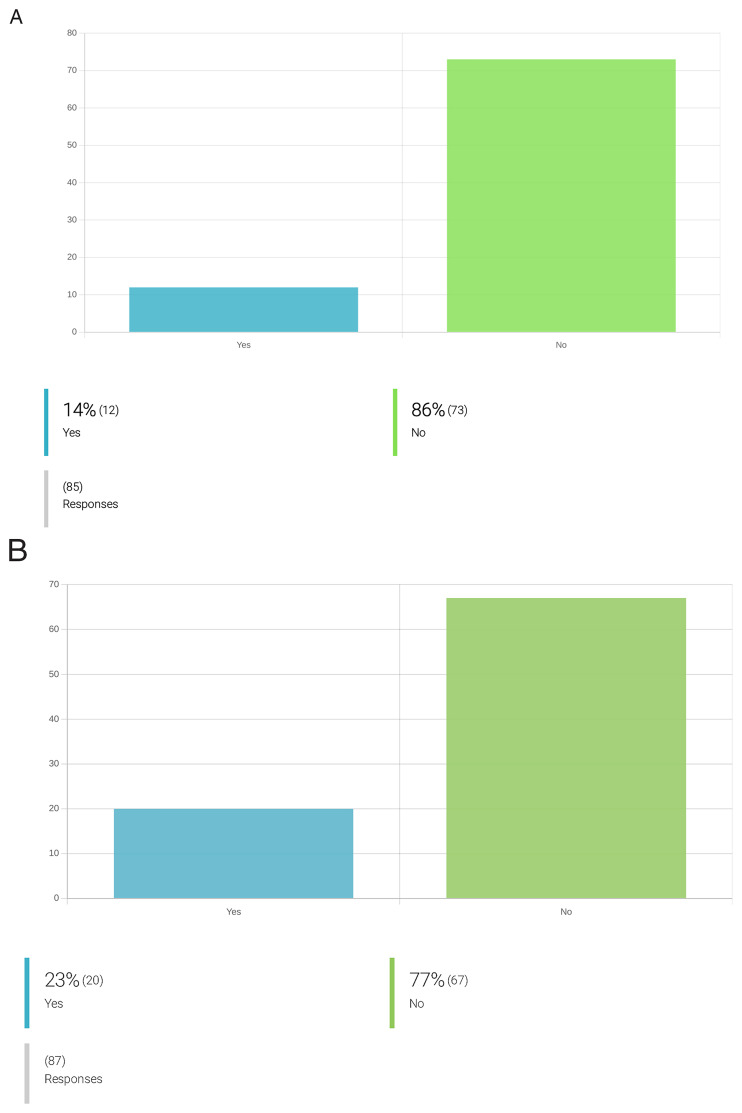
Figure 6. Attitudes towards integration of ecotoxicity and biodegradability assays in newly discovered lead compounds against PVBD.
A. Integration of ecotoxicity assays for new compounds during drug development for PVBDs. B. Integration of biodegradability assays for new compounds during drug development for PVBDs. From the inquired researchers working on drug development for PVBDs, only 14% of the respondents claimed to include ecotoxicity prediction assays in the drug discovery pipeline. The most adopted organisms and models to address ecotoxicity include: testing ecotoxicity towards C. elegans, soil organisms, grass, mammalian cells and free-living protists. Regarding biodegradability assessment of a lead compound, 23% of the inquired respondents (n=20/87) already incorporate such assays in the drug discovery pipeline. For instance, a compound’s biodegradability is addressed by some researchers by selecting plant-derived compounds and biocompatible components; introducing functional groups that favor biodegradation; performing in-silico assays for the prediction of biodegradability, further using this information to prioritize target compounds; identifying drug metabolites under biometric conditions; performing in-vitro ADMET studies and exploring compounds previously synthesized by living organism; privileging compounds without halogen that can be degraded to just CO 2 and water, and eventually ammonia, if they contain nitrogen.