Abstract
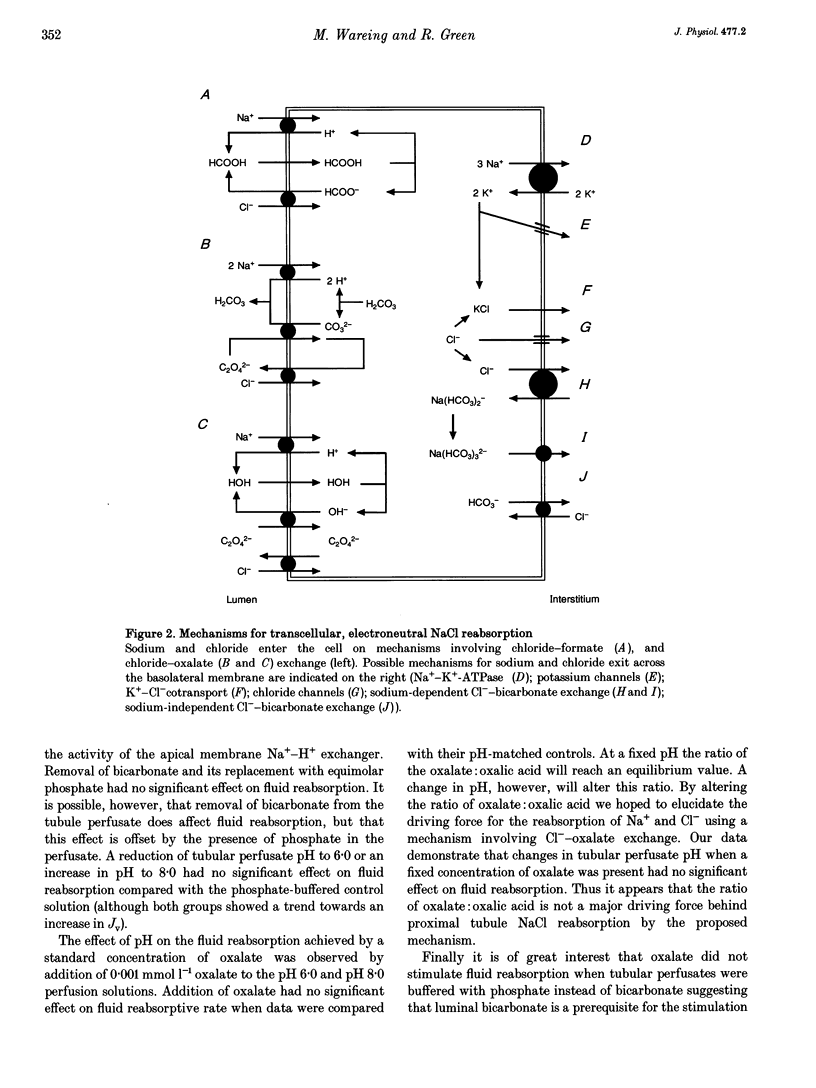
1. It has recently been suggested that both formate and oxalate may be involved in the reabsorption of chloride from the proximal convoluted tubule (PCT). Reabsorption is achieved via a mechanism coupling Na(+)-H+ exchange to Cl(-)-anion exchange thus facilitating net NaCl transport. This possible mechanism was investigated further by observing the effect of luminal addition of formate and oxalate on proximal tubule transport in anaesthetized rats, previously prepared for in vivo microperfusion. 2. Addition of formate (0.25 mmol l-1) to the luminal perfusate resulted in a significant stimulation of fluid reabsorptive rate. 3. Oxalate was found to have a concentration-dependent effect on fluid reabsorption. Luminal oxalate concentrations below 0.05 mmol l-1 stimulated fluid reabsorption, while an oxalate concentration of 0.5 mmol l-1 inhibited fluid reabsorption. 4. The presence of formate (0.25 mmol l-1) and oxalate (0.001 mmol l-1) in the same luminal perfusate stimulated fluid reabsorption from the proximal tubule lumen, but the effects of formate and oxalate were not additive. 5. The removal of bicarbonate from the luminal perfusate and its replacement with phosphate had no significant effect on fluid reabsorptive rate. An alteration in perfusate pH was also ineffective. Addition of oxalate at high and low pH failed to stimulate fluid reabsorption compared with pH-matched control perfusions. 6. The experimental data suggest the involvement of both formate and oxalate in the reabsorption of NaCl from the proximal tubule lumen, though their influence may not be exerted through the same mechanisms.
Full text
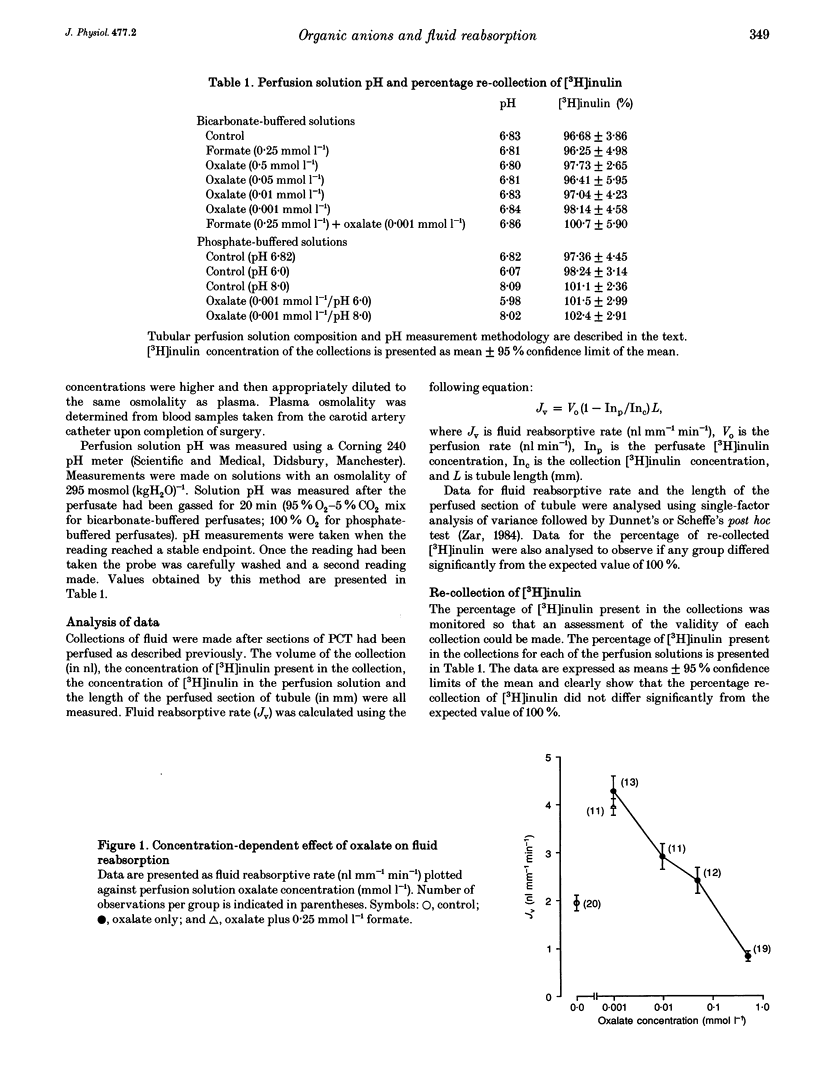
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANNISON E. F. Studies on the volatile fatty acids of sheep blood with special reference to formic acid. Biochem J. 1954 Dec;58(4):670–680. doi: 10.1042/bj0580670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J. Apical membrane chloride/base exchange in the rat proximal convoluted tubule. J Clin Invest. 1987 Apr;79(4):1026–1030. doi: 10.1172/JCI112914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alpern R. J., Cogan M. G., Rector F. C., Jr Flow dependence of proximal tubular bicarbonate absorption. Am J Physiol. 1983 Oct;245(4):F478–F484. doi: 10.1152/ajprenal.1983.245.4.F478. [DOI] [PubMed] [Google Scholar]
- Aronson P. S. The renal proximal tubule: a model for diversity of anion exchangers and stilbene-sensitive anion transporters. Annu Rev Physiol. 1989;51:419–441. doi: 10.1146/annurev.ph.51.030189.002223. [DOI] [PubMed] [Google Scholar]
- Bank N., Aynedjian H. S. Techniques of microperfusion of renal tubules and capillaries. Yale J Biol Med. 1972 Jun-Aug;45(3-4):312–317. [PMC free article] [PubMed] [Google Scholar]
- Baum M. Effect of luminal chloride on cell pH in rabbit proximal tubule. Am J Physiol. 1988 May;254(5 Pt 2):F677–F683. doi: 10.1152/ajprenal.1988.254.5.F677. [DOI] [PubMed] [Google Scholar]
- Baum M. Evidence that parallel Na+-H+ and Cl(-)-HCO3-(OH-) antiporters transport NaCl in the proximal tubule. Am J Physiol. 1987 Feb;252(2 Pt 2):F338–F345. doi: 10.1152/ajprenal.1987.252.2.F338. [DOI] [PubMed] [Google Scholar]
- Burnham C., Munzesheimer C., Rabon E., Sachs G. Ion pathways in renal brush border membranes. Biochim Biophys Acta. 1982 Mar 8;685(3):260–272. doi: 10.1016/0005-2736(82)90066-9. [DOI] [PubMed] [Google Scholar]
- Chantrelle B. M., Cogan M. G., Rector F. C., Jr Active and passive components of NaCl absorption in the proximal convoluted tubule of the rat kidney. Miner Electrolyte Metab. 1985;11(4):209–214. [PubMed] [Google Scholar]
- Chen P. Y., Illsley N. P., Verkman A. S. Renal brush-border chloride transport mechanisms characterized using a fluorescent indicator. Am J Physiol. 1988 Jan;254(1 Pt 2):F114–F120. doi: 10.1152/ajprenal.1988.254.1.F114. [DOI] [PubMed] [Google Scholar]
- France N. C., Holland P. T., McGhie T. K., Wallace M. R. Measurement of plasma oxalate by capillary gas chromatography and its validation by isotope dilution mass spectrometry. J Chromatogr. 1988 Dec 9;433:1–7. doi: 10.1016/s0378-4347(00)80579-4. [DOI] [PubMed] [Google Scholar]
- Fry I. D., Starkey B. J. The determination of oxalate in urine and plasma by high performance liquid chromatography. Ann Clin Biochem. 1991 Nov;28(Pt 6):581–587. doi: 10.1177/000456329102800607. [DOI] [PubMed] [Google Scholar]
- Green R., Bishop J. H., Giebisch G. Ionic requirements of proximal tubular sodium transport. III. Selective luminal anion substitution. Am J Physiol. 1979 Mar;236(3):F268–F277. doi: 10.1152/ajprenal.1979.236.3.F268. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Osmotic forces driving water reabsorption in the proximal tubule of the rat kidney. Am J Physiol. 1989 Oct;257(4 Pt 2):F669–F675. doi: 10.1152/ajprenal.1989.257.4.F669. [DOI] [PubMed] [Google Scholar]
- Green R., Giebisch G. Reflection coefficients and water permeability in rat proximal tubule. Am J Physiol. 1989 Oct;257(4 Pt 2):F658–F668. doi: 10.1152/ajprenal.1989.257.4.F658. [DOI] [PubMed] [Google Scholar]
- Green R., Greenwood S. L., Giebisch G. The role of anions in the regulation of proximal tubular sodium and fluid transport. Ann N Y Acad Sci. 1980;341:125–133. doi: 10.1111/j.1749-6632.1980.tb47167.x. [DOI] [PubMed] [Google Scholar]
- Green R., Windhager E. E., Giebisch G. Protein oncotic pressure effects on proximal tubular fluid movement in the rat. Am J Physiol. 1974 Feb;226(2):265–276. doi: 10.1152/ajplegacy.1974.226.2.265. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Boulpaep E. L., Giebisch G. Electrical properties of chloride transport across the necturus proximal tubule. J Membr Biol. 1982;65(3):185–196. doi: 10.1007/BF01869962. [DOI] [PubMed] [Google Scholar]
- Ives H. E., Chen P. Y., Verkman A. S. Mechanism of coupling between Cl- and OH- transport in renal brush-border membranes. Biochim Biophys Acta. 1986 Dec 1;863(1):91–100. doi: 10.1016/0005-2736(86)90390-1. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Anion exchange pathways for Cl- transport in rabbit renal microvillus membranes. Am J Physiol. 1987 Sep;253(3 Pt 2):F513–F521. doi: 10.1152/ajprenal.1987.253.3.F513. [DOI] [PubMed] [Google Scholar]
- Karniski L. P., Aronson P. S. Chloride/formate exchange with formic acid recycling: a mechanism of active chloride transport across epithelial membranes. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6362–6365. doi: 10.1073/pnas.82.18.6362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura G., Spring K. R. Transcellular and paracellular tracer chloride fluxes in Necturus proximal tubule. Am J Physiol. 1978 Dec;235(6):F617–F625. doi: 10.1152/ajprenal.1978.235.6.F617. [DOI] [PubMed] [Google Scholar]
- Kolb H. A., Brown C. D., Murer H. Identification of a voltage-dependent anion channel in the apical membrane of a Cl(-)-secretory epithelium (MDCK). Pflugers Arch. 1985 Mar;403(3):262–265. doi: 10.1007/BF00583597. [DOI] [PubMed] [Google Scholar]
- Lucci M. S., Warnock D. G. Effects of anion-transport inhibitors on NaCl reabsorption in the rat superficial proximal convoluted tubule. J Clin Invest. 1979 Aug;64(2):570–579. doi: 10.1172/JCI109495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann K. H., Rector F. C., Jr Mechanism of NaCl and water reabsorption in the proximal convoluted tubule of rat kidney. J Clin Invest. 1976 Nov;58(5):1110–1118. doi: 10.1172/JCI108563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrarulo M., Bianco O., Marangella M., Pellegrino S., Linari F., Mentasti E. Ion chromatographic determination of plasma oxalate in healthy subjects, in patients with chronic renal failure and in cases of hyperoxaluric syndromes. J Chromatogr. 1990 Jul 6;511:223–231. doi: 10.1016/s0021-9673(01)93286-8. [DOI] [PubMed] [Google Scholar]
- Preisig P. A., Alpern R. J. Contributions of cellular leak pathways to net NaHCO3 and NaCl absorption. J Clin Invest. 1989 Jun;83(6):1859–1867. doi: 10.1172/JCI114092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preisig P. A., Rector F. C., Jr Role of Na+-H+ antiport in rat proximal tubule NaCl absorption. Am J Physiol. 1988 Sep;255(3 Pt 2):F461–F465. doi: 10.1152/ajprenal.1988.255.3.F461. [DOI] [PubMed] [Google Scholar]
- SONNENBERG H., DEETJEN P. METHODE ZUR DURCHSTROEMUNG EINZELNER NEPHRONABSCHNITTE. Pflugers Arch Gesamte Physiol Menschen Tiere. 1964 Jan 30;278:669–674. [PubMed] [Google Scholar]
- Sabolić I., Burckhardt G. Proton pathways in rat renal brush-border and basolateral membranes. Biochim Biophys Acta. 1983 Oct 12;734(2):210–220. doi: 10.1016/0005-2736(83)90119-0. [DOI] [PubMed] [Google Scholar]
- Schild L., Aronson P. S., Giebisch G. Effects of apical membrane Cl(-)-formate exchange on cell volume in rabbit proximal tubule. Am J Physiol. 1990 Mar;258(3 Pt 2):F530–F536. doi: 10.1152/ajprenal.1990.258.3.F530. [DOI] [PubMed] [Google Scholar]
- Schild L., Giebisch G., Karniski L. P., Aronson P. S. Effect of formate on volume reabsorption in the rabbit proximal tubule. J Clin Invest. 1987 Jan;79(1):32–38. doi: 10.1172/JCI112803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz G. J. Absence of Cl- -OH- or Cl- -HCO3- exchange in the rabbit renal proximal tubule. Am J Physiol. 1983 Oct;245(4):F462–F469. doi: 10.1152/ajprenal.1983.245.4.F462. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Aronson P. S. Cl- transport via anion exchange in Necturus renal microvillus membranes. Am J Physiol. 1984 Dec;247(6 Pt 2):F888–F895. doi: 10.1152/ajprenal.1984.247.6.F888. [DOI] [PubMed] [Google Scholar]
- Seifter J. L., Knickelbein R., Aronson P. S. Absence of Cl-OH exchange and NaCl cotransport in rabbit renal microvillus membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F753–F759. doi: 10.1152/ajprenal.1984.247.5.F753. [DOI] [PubMed] [Google Scholar]
- Shiuan D., Weinstein S. W. Evidence for electroneutral chloride transport in rabbit renal cortical brush border membrane vesicles. Am J Physiol. 1984 Nov;247(5 Pt 2):F837–F847. doi: 10.1152/ajprenal.1984.247.5.F837. [DOI] [PubMed] [Google Scholar]
- Turnberg L. A., Bieberdorf F. A., Morawski S. G., Fordtran J. S. Interrelationships of chloride, bicarbonate, sodium, and hydrogen transport in the human ileum. J Clin Invest. 1970 Mar;49(3):557–567. doi: 10.1172/JCI106266. [DOI] [PMC free article] [PubMed] [Google Scholar]


