Abstract
BACKGROUND
Rhodnius neivai, a kissing bug found in the dry regions of Colombia and Venezuela, has limited documented occurrences. While it is not deemed a significant vector for Chagas disease, distributional and ecological studies are essential in monitoring species domiciliation and shedding light on the evolutionary aspects of the Rhodniini tribe.
OBJECTIVES
The study aims to provide a detailed revision of R. neivai distribution and evaluate general spatial data quality for ecological niche modelling (ENM). It will also provide the first published ENM for the species, which may aid species sampling and future analytical improvement.
METHODS
Registers and other spatial information were gathered by literature review; data georeferencing, preliminary geographical investigations, and model editing were conducted in GIS platforms; ENMs were built using R and explored the uncertainty of parameters and algorithms.
FINDINGS
Twenty four unique sites were identified, unearthing 17 previously uncovered records. Data lacks robust spatial and temporal precision; however, ENMs had acceptable validations. The models present some variation in suitability but with objective areas for sampling effort.
MAIN CONCLUSIONS
Rhodnius neivai distribution is better explained by conditions that characterise dry ecotypes, but further sampling is essential to improve modelling and advance with ecological and evolutive matters.
Key words: Rhodnius neivai, Rhodniini, ecological niche model, Chagas disease, neotropics, South America
Triatomines are insects that belong to the Triatominae subfamily (Hemiptera: Reduviidae), known by their blood-sucking habit and to be the vectors of Trypanosoma cruzi Chagas, 1909, the causative agent of Chagas disease. 1 Rhodnius neivai Lent, 1953 is a species within the Rhodniini tribe, composed of 23 species grouped in the Rhodnius Stål, 1859, and Psammolestes Bergroth, 1911, genera, being the second most diverse of the Triatominae tribes. 2 , 3 , 4 , 5 R. neivai has been found in Venezuela and Colombia, 6 , 7 where kissing bugs are commonly known by the local population as pito or chinche picuda and chipo, respectively. 1
Most observations of R. neivai have occurred in dry ecotypes, 8 with register in palms, trunks of dead trees, and inside and around domiciles; 9 , 10 however, there is no evidence to suggest that colonies are maintained in houses. 11 While R. neivai sylvatic habitat and low resistance to starvation suggest the species’ preference for non-human feeding sources and easier epidemiological control, there is a recognised need for surveillance due to the species’ natural T. cruzi infection, the capacity to feed on humans, birds and rodents’ blood, aggressive behavior and to its potential for domiciliation. 6 , 12
Rhodnius prolixus has historically been the most relevant vector of Chagas in Colombia and Venezuela. 13 However, outbreaks of the disease in the acute stage have more recently been associated with oral transmission and with the increased relevance of secondary vectors in the region. 14 , 15 R. neivai findings were not reported in these surveys, but further knowledge of vector biology may be a cautious measure for new epidemiological scenarios.
Spatial data and geographical information system (GIS)-based analyses have played an essential role in epidemiological surveillance efforts, including those for vector-borne diseases. 16 , 17 In recent years, ecological niche models (ENMs) have gained prominence in studying triatomines in public health 18 , 19 , 20 and for historical biogeographical research. 21 , 22
Limited species records 23 , 24 and poor geographical precision 25 , 26 often constrain the development of robust ENMs. This is particularly true for kissing bugs that inhabit sylvatic areas and poses a lower risk in the T. cruzi epidemiological cycle involving humans; these species generally suffer from insufficient distributional data and heightened spatial sampling bias. 27 Such is the case for R. neivai - few occurrence points are available, both in works with geopolitical reviews of triatomine distribution 6 , 7 , 28 and the database of American triatomine species occurrence - DataTri. 29 , 30
New geographical finds for rare species are essential for overcoming macroscale biodiversity shortfalls, as information gaps are based on species distribution (Wallecean shortfall) and evolutive relations (Darwinian shortfall). 31 Species limit samples are a recognised barrier for evolutive and biogeographical studies within Triatominae. 3 Including further data in the phylogenetical analysis has provided new taxonomical relations for Reduviidae, 5 which corroborates the relevance of applying efforts to find rare species. For biogeographical matters, R. neivai new findings may be relevant in the exploration of historical connectivity between its dry occurrence region and the Atlantic Forest 22 to test the niche conservationism hypothesis and distributional range shift due to climate change.
In this study, we review and update the distributional information available in the scientific literature for R. neivai, supplementing it with additional georeferenced records for the species. While the data we obtained were temporally biased and suffered from low geographical precision, they were sufficient to produce the species’ first published ENMs. A model identified Annual Precipitation as the most critical predictive variable for explaining the species distribution and provides a foundation for guiding future sampling efforts in known distributional areas.
MATERIALS AND METHODS
Occurrence data gathering and georeferencing - We revised the spatial information for R. neivai from older literature in BibTri version 3.0 (Centro de Estudios Parasitológicos y de Vectores - CEPAVE) 32 and in specialists’ libraries to more recent online publications. We also checked DataTri records 29 , 30 available in the Global Biodiversity Information Facility (GBIF) 33 for data incorporation in our analyses and discussion. For georeferencing species records, we used the centroids of the most precise correspondent geopolitical units available in maps made available by the United Nations Office for Coordination of Humanitarian Affairs (UNOCHA). 34 Register based only on available published maps were georeferenced, overlapping the maps with species occurrence indication and known geographical information. If the geopolitical unit, at least at the municipality level, was unavailable in the previously cited formats, we used the coordinates from Google Maps (https://www.google.com/maps). Geoprocessing used the World Geodetic System 1984 (WGS84) datum in ArcMap V.10.8. 35
Georeferenced R. neivai occurrence data was plotted over the available grid of biogeography provinces built by combining climatic, geological, and biotic criteria with areas of endemism. 36 We also plotted the register on top of widely accepted ecoregions proposed for the world, claiming to reflect better species and communities’ distribution of biophysical features. 37 These units may not represent the precise distribution of the R. neivai in their delimitations but can provide hints on geographical characteristics and areas of relevance for the species.
Ecological niche models - ENMs were first built following a similar combination approach and the same environmental data previously applied for Rhodnius domesticus Neiva and Pinto, 1923 in its calibration area: 22 We test the same 17 bioclimatic environmental layers in the resolutions of 5 arc minutes from Climatologies at high resolution for the Earth’s land surface areas (CHELSA) in variables choice, available at PaleoClim. 38 , 39 , 40 The bioclimatic environmental variables Precipitation of Coldest Quarter (Bio 19), Precipitation of Warmest Quarter (Bio 18), Mean Temperature of Driest Quarter (Bio 9), and Mean Temperature of Wettest Quarter (Bio 8) were indicated to present distortions in particular study areas and have been discarded in previous ENMs. 41 , 42 , 43 , 44 As a priori all 19 variables could be equally relevant for ENMs, we exclude the ones with observed odd discontinued spatial distribution. 45 Only Bio 18 and Bio 19 were deleted since no other variable presented the spatial artifact. See Supplementary data (253.9KB, pdf) (Text, Fig. 1) for information on further variables.
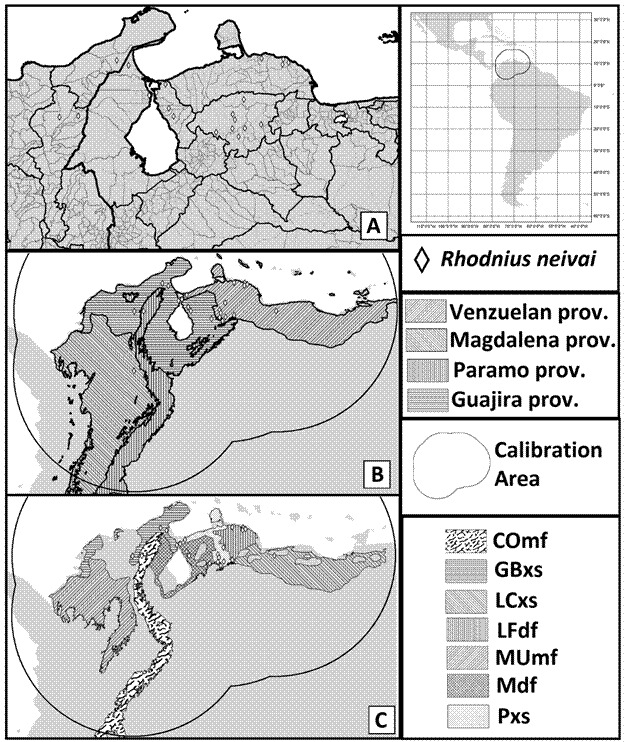
Fig. 1: maps of Rhodnius neivai geographical occurrences and related geographical relevant areas; register for the R. neivai are indicated as a lozenge, the figure in the right upper corner indicates the neotropical region and surrounding areas, the circular area indicates the calibration area used in ecological niche modeling. (A) Geopolitical delimitations of Colombia and Venezuela, where the species has been found, darker and thicker lines indicate higher-level political administrations and clear and thinner lines indicate lower-level administrations. (B) Biogeographical provinces 36 related to species occurrences indicated by different stripes angulations: Venezuelan (45 degrees); Magdalemo (-45 degrees); Paramo (90 degrees); Guajira (180 degrees). (C) Ecoregions 37 related to species occurrences indicated by acronyms and different textures: Cordillera Oriental montane forests (COmf - “cowhide” pattern); Guajira-Barranquilla xeric scrub (GBxs - 180-degree stripes); La Costa xeric shrublands (LCxs - -45 degree stripes); Lara-Falcón dry forests (LFdf - 90-degree stripes); Magdalena-Urabá moist forests (MUmf - 45-degree stripes); Maracaibo dry forests (Mdf - checkered pattern); Paraguana xeric scrub (Pxs - dots and white background).
ENMs were produced using Maxent version 3.4 in R. 46 , 47 The calibration area was defined by a five-decimal degree buffer around occurrence points, 22 , 48 and we used the KUENM package 49 for the combination of predictive variables and Maxent parameters, models building, and evaluations. We built candidate models based on 70% occurrence data and 30% testing, three predictor variables (from all the 17 possible ones), with highly correlated sets of variables being excluded (|r| > 0.8) to deal with collinearity issues 50 and data processing limitation. 22 , 51 The correlation was calculated using the SDM toolbox 52 in ArcMap V.10.8. 34 We applied the linear, quadratic, and product feature classes and the following regularisation multipliers, setting applied in exhausting modelling approaches: 53 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, 0.7, 0.8, 0.9, 1, 2, 3, 4, 5, 6, 8, 10.
Candidate models were evaluated by statistical significance using partial receiver-operating characteristic curve (ROC), with applied omission-based parameter E used in ROC curve delimitations (E = 20%; 500 iterations and 50% of data for bootstrapping) and Akaike’s information criterion (AICc) criteria. 49 , 54 Omission by the corresponding threshold for E was not used in the evaluation, given the low amount of occurrence data and a single run of training and testing sampling used in candidate model building. Since we have a low number of records, any single absence in testing omission would be highly relevant, and distinct sampling for training and testing may select distinct ENMs. We choose the set of variables and parameters from the best candidate model according to AICc criteria and produce the final ENM using cross-validation for the sampling test with replications equal to the number of occurrences and background points equal to the number of pixels in the calibration area. The final ENM was the average ENM related to the training samples. Despite the valid critical points made regarding AUC in model evaluation, 55 we also indicated the mean values from the Maxent output to inform a more general trend of validation in our relatively concise study area.
From the final average model, predictive variables percentage contribution (PC) and permutation importance (PI) in ENM were evaluated to indicate the climatic conditions that better explain species distribution. We also create a categorical model summing two binary ENMs based on the 10-percentile and minimum training presence. As the less restrictive threshold comprehends all pixels of the more restrictive one, the final model indicates three areas: absence of suitability, suitable areas with a higher risk of commission error, and more restrictive areas for species suitability. This model allows objective regions for species sampling efforts to consider two distinct levels of exploration: areas more likely to find the species and more unusual areas where species may occur.
To address the matters of different algorithm responses 56 and to incorporate Principal Component Analysis (PCA) as an alternative approach to deal with predictive variables collinearity, 57 a second set of models was produced using the same 17 spatial delimited variables and the following algorithms: Bioclim, Generalises Additive Model, Generalised Linear Model, Maxent and Random Forest. Background points were randomly generated, and model evaluation was conducted by AUC, Kappa, TSS, Jaccard, and Sorensen using the K-Fold (K = 4) data partition method based on the Least Presence Threshold. Models presenting final scores under 0.7 were not taken into consideration. Binary models and omission rates were calculated using the same threshold. For those models, all processing steps were taken using the ENMTML package in R. 58 The final average, the sum of binary models, and standard deviation models were produced in ArcMap V.10.8 raster calculator. 35
Ethics - The present work was based entirely on secondary triatomine (invertebrate organism) occurrence data gathered by literature review and open data sets, compiled according to all regulations.
RESULTS
Occurrence data gathering and georeferencing - Our revision found 24 registered sites for R. neivai in Venezuela and Colombia (Table, Fig. 1A). Only six records indicated the sampling date; none provided precise geographic coordinates. Our list of occurrence sites includes the seven correspondent registers available in DataTri 29 , 30 and adds 17 uncovered registers from older Venezuelan literature. 9 , 59 , 60 , 61 , 62 , 63 Some distinct nominal geopolitical information and coordinates differ between the cited literature centroids 28 , 64 and DataTri [Supplementary data (253.9KB, pdf) (Table I)]. The divergence of spatial data must be related to distinct georeferencing and spatial geopolitical indication strategies and possible further information provided by specialists to DataTri curators. 29 , 30
TABLE. Compilation of spatial information related to Rhodnius neivai geographical distribution.
| IDR | IDS | Source | S.Date | Country | State | Municipality | Locality | GeoUnit | Latitute | Longitude | Province | Ecoregion |
| 1 | 1 | Lent and Wygodzinsky, 1979 | 1968 | Colombia | Cesar | Valledupar | - | Valledupar | 10.2189 | -73.4578 | Guajira | GBxs |
| 2 | 2 | Morales et al., 1987 | 1982 | Colombia | La Guajira | Maicao | Calabacito | Maicao | 11.3816 | -72.2950 | Guajira | GBxs |
| 3 | 3 | Guhl et al., 2007 | <= 2007 | Colombia | Cesar | La Paz | - | La Paz | 10.2444 | -73.0782 | Paramo | COmf |
| 4 | 4 | Guhl et al., 2007 | <= 2007 | Colombia | Cesar | San Alberto | - | San Alberto | 7.7698 | -73.4722 | Magdalena | Mumf |
| 5 | 1 | Guhl et al., 2007 | <= 2007 | Colombia | Cesar | Valledupar | - | Valledupar | 10.2189 | -73.4578 | Guajira | GBxs |
| 6 | 5 | Guhl et al., 2007 | <= 2007 | Colombia | Magdalena | - | Sierra Nevada de Santa Marta | - | ? | ? | ? | ? |
| 7 | 6 | Lent, 1953 | 1951 | Venezuela | Lara | Camacaro | caserio Parapara | Camacaro | 10.2816 | -69.9333 | Venezuelan | Pxs |
| 8 | 7 | División de Endemias Rurales, 1965 | 1962-1964 | Venezuela | Falcon | - | - | P.MAP | 11.4008 | -68.8960 | Venezuelan | LFdf |
| 9 | 8 | División de Endemias Rurales, 1965 | 1962-1964 | Venezuela | Falcon | - | - | P.MAP | 11.2958 | -68.9135 | Venezuelan | LFdf |
| 10 | 9 | División de Endemias Rurales, 1965 | 1962-1964 | Venezuela | Falcon | - | - | P.MAP | 11.3351 | -68.7943 | Venezuelan | LFdf |
| 11 | 10 | Lent and Juberg, 1969 | <= 1996 | Venezuela | Lara | Antonio Díaz | caserio Las Playas | Antonio Díaz | 9.9852 | -69.9367 | Venezuelan | Pxs |
| 12 | 11 | Veliz et al., 1972 | <= 1972 | Venezuela | Lara | Barquisimeto | - | Barquisimeto | 10.0677 | -69.3473 | Venezuelan | Pxs |
| 13 | 12 | Otero et al. 1975ab | <= 1975 | Venezuela | Falcon | Puerto Cumarebo | - | Puerto Cumarebo | 11.4197 | -69.3423 | Venezuelan | LFdf |
| 14 | 13 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Juan Bautista Rodriguez | - | Juan Bautista Rodriguez | 9.9964 | -69.6844 | Venezuelan | Pxs |
| 15 | 14 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Bolivar | - | Bolivar | 9.8235 | -69.8073 | Venezuelan | Pxs |
| 16 | 10 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Antonio Díaz | - | Antonio Díaz | 9.9852 | -69.9367 | Venezuelan | Pxs |
| 17 | 15 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Espinoza de Los Monteros | - | Espinoza de Los Monteros | 10.1688 | -69.8864 | Venezuelan | Pxs |
| 18 | 16 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Manuel Morillo | - | Manuel Morillo | 9.9140 | -70.2729 | Guajira | Mdf |
| 19 | 17 | Otero et al. 1975ab | <= 1975 | Venezuela | Lara | Siquisique | - | Siquisique | 10.5798 | -69.7056 | Venezuelan | Pxs |
| 20 | 18 | Otero et al. 1975ab | <= 1975 | Venezuela | Zulia | Cabimas | - | Cabimas | 10.4057 | -71.2308 | Guajira | Mdf |
| 21 | 19 | Otero et al. 1975ab | <= 1975 | Venezuela | Zulia | Lagunillas | - | Lagunillas | 10.2375 | -71.1316 | Guajira | Mdf |
| 22 | 20 | Otero et al. 1975ab | <= 1975 | Venezuela | Zulia | Altagracia | - | Altagracia | 10.7259 | -71.4858 | Venezuelan | Pxs |
| 23 | 21 | Carcavallo et al. 1976 | <= 1976 | Venezuela | Zulia | Goajira | - | Goajira | 11.2765 | -72.0522 | Guajira | GBxs |
| 24 | 22 | Carcavallo et al. 1976 | <= 1976 | Venezuela | Zulia | Faria | - | Faria | 10.8897 | -71.2989 | Venezuelan | Pxs |
| 25 | 23 | de Olaria, 1985 | <= 1985 | Venezuela | Zulia | Chinquinquirá | sector Delicias | Chinquinquirá | 10.6664 | -71.6315 | Guajira | GBxs |
| 26 | 24 | Harry et al., 2008 | <= 2008 | Venezuela | Aragua | Maracay | - | Maracay | 10.2442 | -67.6066 | Venezuelan | LCxs |
| 27 | 24 | Pita et al., 2013 | <= 2013 | Venezuela | Aragua | Maracay | - | Maracay | 10.2442 | -67.6066 | Venezuelan | LCxs |
IDR: code for literature; IDS: code for georeferenced site; Source: literature reference; S date: sampling date; GeoUnit: spatial unit georeferenced; Province: biogeographical units; 36 Ecoregions 37 names and correspondent acronyms: Cordillera Oriental montane forests [COmf]; Guajira-Barranquilla xeric scrub [GBxs]; La Costa xeric shrublands [LCxs]; Lara-Falcón dry forests [LFdf]; Magdalena-Urabá moist forests [MUmf]; Maracaibo dry forests [Mdf]; Paraguana xeric scrub [Pxs]).
All sites were used in the ENM and overlapping analyses except for the record related to the Sierra Nevada de Santa Marta, which we could not georeferenced given the lack of geopolitical unit indication. Coordinates for the datum seem to be available in DataTri; however, they do not match the geopolitical information indicated in the species literature. 7 , 28 We prefer not to provide centroids for a larger mountainous area or use coordinates without explicitly confirming their occurrence due to the potential for significant variations of climate conditions in short geographical ranges. 65
Concerning the areas proposed in Neotropical regionalism, 36 R. neivai records were mainly comprised in regions of Venezuelan and Guajira provinces, with fourteen and seven occurrences, respectively (Table, Fig. 1B). Both provinces of Paramo and Magdalena were related to a single record. For the ecoregions 37 registers were more common in areas defined by dry conditions (Table, Fig. 1C): Paraguana xeric scrub (10 registers); Guajira-Barranquilla xeric scrub (four registers); Lara-Falcón dry forests (four registers); Maracaibo dry forests (three registers); La Costa xeric shrublands (one register); Cordillera Oriental montane forests (one register); and Magdalena-Urabá moist forests (one register). It is essential to notice that other strategies or more precise information in georeferencing may indicate differences in spatial relations between the records and provinces or ecoregions.
Ecological niche models - From our combination of the 17 predictive variables in a set of three and the deletion of sets that contain highly correlated variables (|r| > 0.8 :: correlation matrix available in Supplementary data (253.9KB, pdf) (Table II), 347 sets of environmental variables remain. A total of 17,697 candidate models were produced from the combination of environmental sets, three feature classes, and 17 regularisation multipliers. The evaluation process indicates 85 statistically significant models following AICc criteria based on 13 selected environmental variables. The model with the best validation was based on a 0.1 regularisation multiplier, quadratic feature class, and the following predictive variables with their correspondent variable contribution (PC) and impact (PI): annual precipitation (Bio 12 :: PC = 86.1; PI = 79.5); precipitation seasonality (Bio 15 :: PC = 10.9; PI = 16); and max temperature of warmest month (Bio 5 :: PC = 3; PI = 4.5).
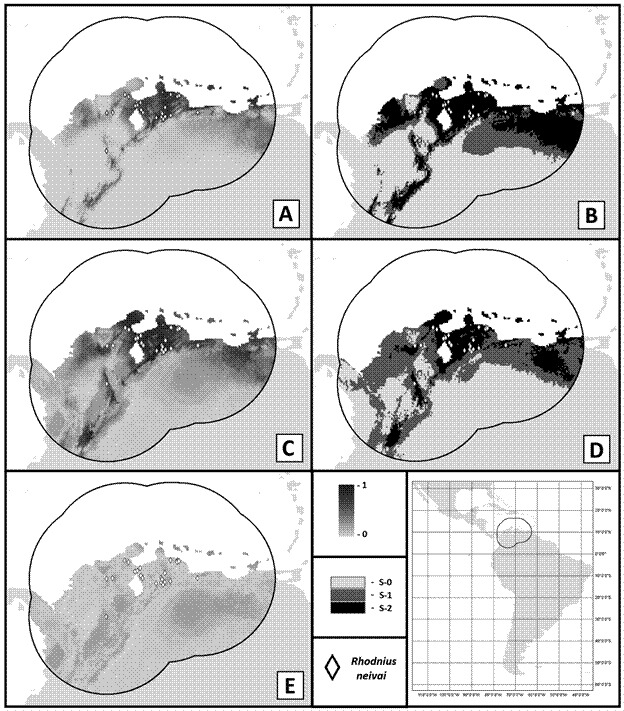
The final ENM was built using 23 occurrence points; therefore, as previously stipulated, 23 repetitions for cross-validation and background points equals 13,663 pixels, and the test AUC was equal to 0.898. Suitability was higher in dryer northern parts of the study area, with values varying between practically zero to near 0.87 (Fig. 2A). Threshold values for the 10-percentile training presence equals 0.1537, and for the minimum training presence, 0.0319. The categorical model also indicates the most restrictive area for suitable conditions in the north and less restrictive in its surroundings, being more significant in the eastern part of the map (Fig. 2B).
Fig. 2: ecological niche models (ENMs) for Rhodnius neivai; register for the species are indicated as a lozenge, the figure in the left-down corner indicates the neotropical region and surrounding areas, the circular area indicates the calibration area for the ENMs; note the analyses were only made for this particular geographical region. On the left column are present continuous maps with float values, ranging from zero (lighter gray) to one (darker gray). Maps in the right column are derived from the sum of binary models and present categories: S-0 (no suitability), S-1 (suitability given one scenario), and S-2 (suitability given both scenarios). (A) Three variables ENM. (B) Categorical model based on the sum of binary three variables ENMs, scenarios are based on thresholds. (C) Principal Component Analysis (PCA) variables ENM. (D) Categorical model based on the sum of binary PCA ENMs, with scenarios, based on different algorithms. (E) Standard deviation model for PCA variables ENM, based on algorithm variation.
For the alternative model, PCA produced five distinct variables, the model omission rate was always equal to zero, and from further model validation, only Maxent and Random Forest present scores above 0.7 [see Supplementary data (253.9KB, pdf) (Table III, Fig. 2) for complete model validation and individual ENMs, respectively]:
Maxent - AUC = 0.8042; Kappa = 0.7417; TSS = 0.7417; Jaccard = 0.7976; Sorensen = 0.8866. The continuous model presented a visual aspect closer to the previous one, which was made with only three variables. However, the binary version had a more extensive area indicating species presence [Supplementary data (253.9KB, pdf) (Fig. 2)].
Random Forest - AUC = 0.8250; Kappa = 0.7417; TSS = 0.7417; Jaccard = 0. 8036; Sorensen = 0.8888. The continuous model visually presents a larger suitability area; however, the values seemed generally lower. The binary model indicates the smallest area for the models [Supplementary data (253.9KB, pdf) (Fig. 2)].
Both average models presented somewhat similar suitability distribution, with this alternative model possessing more intermediary values (Fig. 2C). In the binary model, a larger suitable region appears west of the study area. However, the divergence between algorithm outputs is larger, making a visually smaller area of consensus (Fig. 2D). The standard deviation model indicates a significant extent with values up to 0.309 of variation, which include areas of high suitability (Fig. 2E).
DISCUSSION
Historical sampling for R. neivai - We provide detailed information on the sampling history for R. neivai in this section, given the rare records for the species, starting from its description: R. neivai was first collected in 1951 by Suárez in the Locality of caserío Parapara, Camacaro, state of Lara, Venezuela, then was send through Gabaldon to Lent that described the species. 66 , 67 The type specimen was indicated to be deposited in the entomological collection of the Oswaldo Cruz Institute (CT-IOC) in Rio de Janeiro, Brazil. 64 , 66
An official Venezuelan campaign against Chagas diseases report acknowledges the occurrence of R. neivai in Lara. It indicates that what seems to be three records for R. neivai in the state of Falcón were found in houses between 1962 and 1964, but the localities’ or municipalities’ names were not provided. Still, the distribution in the country map was made available. 59 According to our georeferencing, all points are contained in the geopolitical delimitations of the locality of San José de la Costa, Municipality of Piritu.
A genitalia study of the Rhodnius genera indicated an allotype from the locality of caserío Las Playas, Municipality of Antonio Diaz, state of Lara. 68 The species was also infected with T. cruzi in Barquisimeto, state of Lara, Venezuela. 6 , 60 Later, the work acknowledges the species distribution in Lara and Falcón in a small table with species identification made up to 1959 and 1965, respectively, but does not provide precise geopolitical information and strangely indicates in the main text that R. neivai to be only captured once in the state of Lara. 69
In dry regions of Puerto Cumarebo, Falcón, Venezuela, Rhodnius brethesi Matta, 1919 was registered, a triatomine typical of wet Amazon areas; however, taxonomical revision indicated that specimens were R. neivai. 61 , 62 The same authors also made new registers in the state of Zulia: Cabinas; Lagunillas; and Altagracia; and in Lara: Sisquisique; Juan Bautista Rodriguez; Bolivar; Antonio Diáz; Espinoza de Los Monteros; and Manuel Morillo. 61 A World Health Organization (WHO) report 62 provided identical records, except for Espinoza de Los Monteros, which was indicated as “E. de los Rios”. No mention of the registers we georeferenced to be in San José de la Costa was made after the campaign report, 59 making the records somewhat dubious.
Sylvatic field searches for triatomines made in the state of Zulia register R. neivai in Faría on a dry tree and in Goajira (georeferenced by us as Guajira) associated with palm. 9 Actualisation of triatomine distribution according to biogeographical zonation indicates species distribution related to dry regions in Lara, Falcón, and Zulia. 8 The species was posteriorly found inside a house in el sector Delicias, Chinquinquirá, Maracaibo, in the state of Zulia. 63 Genetical works for the Rhodniini species also indicate R. neivai presence in Maracay, Venezuela, related to specimens deposited in Oswaldo Cruz Institute, Fiocruz Insectary, Rio de Janeiro, Brazil. 70 , 71 We did not find any further registers for the species in Venezuela; its distribution is related to northwest regions in the country (Fig. 1A).
In Colombia, the first register for R. neivai was made in 1968 and found in “Magdalena: Valledupar”; 11 posteriorly, the site was indicated to be comprised in the state of Cesar. 72 The second register in the country was made using artificial lights in the corregimiento de Calabacito, Maicao, state of La Guajira, in 1982. 73 In a review of triatomine distribution in Colombia, new registers were also indicated from the state of Cesar in La Paz, San Alberto, and others for Valledupar and in the state of Magdalena in the Sierra Nevada de Santa Marta. 28 In fieldwork and database assemblage for triatomines in Colombia, it is indicated that R. neivai has not registered in further municipalities since the 2007 published revision for the country. 7 , 28
The known register of R. neivai in Colombia is mainly related to the country’s northern areas (Fig. 1A). The occurrence in San Alberto was by far the most isolated and southern point in all species distribution and is the only one in the biological province of Magdalena and the Magdalena-Urabá moist forests ecoregion. The unusual record may be related to species random dispersion or population related to small available habitats in the region. La Paz’s register is also the single point in Paramo province and Cordillera Oriental montane forests ecoregion. However, this may be related to geospatial imprecision and the narrow dimension of the biogeographical areas.
The record of the Sierra Nevada de Santa Marta is proposed to be comprised in Magdalena. 28 , 30 Still, the most recent compilation on species distribution in Colombia does not acknowledge any of its municipalities for the record. 7 It is also possible that changes in names or delimitations of geopolitical units lead to mismatched spatial information, but we did not evaluate such matters. The short spatial variation of biodiversity and climatic zonation found across mountain elevations 65 , 74 makes high precision on geographical occurrences of R. neivai even more critical to understanding the diversity of climate conditions the species can inhabit.
Ecological niche models - The latitudinal gradient is an essential factor related to triatomine richness. 75 Other macroecological analyses include triatomine richness found in dryer areas in the neotropics and the temperature variables being relevant in explaining species richness and distribution, even though other variables may be relevant to explain a large part of diversity patterns. 76 Our first analyses support the relation of R. neivai occurrences in dry environments 10 but indicate precipitation variables being more relevant in explaining the species distribution. Annual Mean Precipitation contribution and importance were far more relevant than both other two variables combined. This may be explained by the low number of variables used in model building and the low correlation between each other (|r| < 0.8). As further registers are made for the species, more variables may be added to modeling, and more complex environmental responses may be indicated. However, the climate relation is ecologically meaningful, and precipitation variables could remain relevant for future R. neivai models, given that precipitation seasonality was also more relevant than the maximum temperature of the warmest month.
ENMs for Triatoma dimidiata Latreille, 1811 indicate annual mean precipitation as one of Colombia’s most relevant variables for the triatomine distribution. 77 ENMs for other triatomines in the country exclude this variable given their strategy of dealing with variables collinearity; it indicates other precipitation variables with high contribution for the models, often precipitation seasonality being of critical relevance 78 and makes us wonder if precipitation-derived variables may be necessary for distribution in the macro biodiversity scale in the region. For Venezuelan triatomines ENMs, on the other hand, only temperature-derived variables were relevant for the models, 79 and precipitation variables were considered less critical for Rhodnius prolixus Stål, 1859. 80 Some of these applications used study areas based on geopolitical delimitation; applying the species’ total distribution and meaningful ecological calibration areas may provide distinct results for the models. 81
ENMs for triatomines using full distribution and calibration areas based on ecoregions indicate that some precipitation variables were relevant for Triatoma maculata Erichson, 1848 (with register both in Colombia and Venezuela) and Rhodnius pallescens Barber 1932, (with register in Colombia but not in Venezuela), the latter including annual mean precipitation as a significant variable in model building. 51 The trend of variable responses for particular regions is of general biogeographical interest. However, these areas include different environmental regions and distinct species distributions that may indicate specific variables’ contribution and importance in their corresponding models. For example, distinct variables act as limiting factors based on ENMs for different species that also occur in the general region. 82
Despite relevant suitability being present in some humid regions of the study area, the higher spatially cluster values for the first model were mainly in northern dryer parts (Fig. 2A). According to our georeferenced data, Southerner and Westerner suitable areas may represent montane valleys or slopes, and species distribution in those points is the most uncertain. Suitability was found around, but not in, the Sierra Nevada de Santa Marta’s general area, which may indicate the record from lower elevation regions. The Westerner points for R. neivai are from San Alberto, La Paz, and Valledupar. They are only supported by the most unrestricted threshold (Fig. 2B). Species could have dispersed from near suitable areas, or unprecise georeferencing could influence the response. Centroids are points that provide spatial bias when applied in ENMs. 25 , 26 Given ecological coherence in variables and the suitability of spatial distribution, we do not trust that these spatial errors may represent a substantial risk in model response. However, we have a small dataset, so they have become highly relevant in model evaluation, especially regarding omission rate.
The alternative model based on distinct algorithms, PCA variables, and a more inclusive threshold indicates a similar response to continuous (Fig. 2C) and binary (Fig. 2D) derived models. Some larger suitable areas are indicated in westerner regions of Colombia (Fig. 2D). However, the binary divergence between Maxent and Random Forest models (Fig. 2D) and some high areas of standard deviation (Fig. 2E) make the areas less robust. Considering maps available for biomes and ecoregions of Colombia, 83 these newly suitable areas comprise large moist regions that would be less expected for species occurrence. Among the previously discussed biases, this result could be influenced by the more inclusive threshold. As the binary models used PCA variables and the least presence values for threshold, the model will not be highly informative for discussion on the ecological influence of the variable and the occurrence sites that are less robust.
Further sampling for the species with more accurate geospatial information may provide robustness in R. neivai ENMs and supply better temporal data, given the old date of the records (Table). However, we acknowledge the substantial limitations of sampling rare sylvatic triatomines. 27 On the other hand, the effort may prove helpful in exploring interesting biogeographical events in Rhodniini historical biogeography: R. domesticus, proposed to be R. neivai sister species, 84 is related to more distant moist regions in the Atlantic Forest. 18 , 22 , 85 Transferring ENMs for R. neivai may help to explore the climatic range shift effects in species distribution and explain the current distance between species. Well-established niches in environmental space may also help test significant niche divergence in cladogenesis. Niche conservationism was proposed to fit better niche relations between sister species of North and Central America triatomines. 86 However, R. neivai and R. domesticus likely evolutive “niche dissimilarity” may indicate distinct relations in triatomine and South American groups. We trust that to test the hypothesis in the future, it would be required to make occurrence data for both R. domesticus 22 and R. neivai more robust and certify the phylogenetical relation between the species.
In conclusion - As expected, our literature revision indicates that available information on R. neivai distribution is scarce and has low spatial and temporal precision. Nevertheless, the 17 recovered records in Venezuela provide a better occurrence sample for applying ecological niche modelling. Our model indicates annual mean precipitation as the predictive variable that significantly influences the explanation of species distribution. The relation makes ecological sense for R. neivai, given its historical occurrence in dry environments. 10 The ENMs have limitations, and some occurrences for the species may be geographically dislocated from their natural source (the Sierra Nevada de Santa Marta, San Alberto, La Paz, and Valledupar). Our models may aid the field search for R. neivai in a more practical context. Any new record for the species may provide important data for the application of the method, and future sampling must aim to provide high geographical precision; the coordinates for the register would be the most suitable type of information. More complex ENMs and biogeographical hypotheses may be explored in the future as new registers for the species are made.
Funding Statement
FAPES (award number - 011/2019) [The study was derived from the GSCN PhD thesis from the Programa de Pós-Graduação em Biologia Animal (PPGBAN) of UFES, which FAPES financed], CNPq [Since further work improvements, reviews, and submissions were made over GSCN participation in the Programa de Capacitação Institucional (PCI) of the Brazilian Ministry of Science, Technology, and Innovation (MCTI) at the National Institute of the Atlantic Forest (INMA) (CNPq award number - 317350/2023-4)].
Footnotes
Financial support: FAPES (award number - 011/2019) [The study was derived from the GSCN PhD thesis from the Programa de Pós-Graduação em Biologia Animal (PPGBAN) of UFES, which FAPES financed], CNPq [Since further work improvements, reviews, and submissions were made over GSCN participation in the Programa de Capacitação Institucional (PCI) of the Brazilian Ministry of Science, Technology, and Innovation (MCTI) at the National Institute of the Atlantic Forest (INMA) (CNPq award number - 317350/2023-4)].
REFERENCES
- 1.Schofield CJ, Galvão C. Classification, evolution, and species groups within the Triatominae. Acta Trop. 2009;110(2-3):88–100. doi: 10.1016/j.actatropica.2009.01.010. [DOI] [PubMed] [Google Scholar]
- 2.Justi SA, Galvão C. The evolutionary origin of diversity in Chagas disease vectors. Trends Parasitol. 2017;33(1):42–52. doi: 10.1016/j.pt.2016.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Monteiro FA, Weirauch C, Felix M, Lazoski C, Abad-Franch F. Evolution, systematics, and biogeography of the Triatominae, vectors of Chagas disease. Adv Parasitol. 2018;99:265–344. doi: 10.1016/bs.apar.2017.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Oliveira-Correia JPS, Oliveira J, Gil-Santana HR, Rocha DS, Galvão C. Taxonomic reassessment of Rhodnius zeledoni Jurberg, Rocha & Galvão a morphological and morphometric analysis comparing its taxonomic relationship with Rhodnius domesticus Neiva & Pinto. BMC Zool. 2024;9(1):6–6. doi: 10.1186/s40850-024-00197-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Masonick PK, Knyshov A, Gordon ERL, Forero D, Hwang WS, Hoey-Chamberlain R, et al. A revised classification of the assassin bugs (Hemiptera: Heteroptera: Reduviidae) based on combined analysis of phylogenomic and morphological data. Syst Entomol. 2024 [Google Scholar]
- 6.Cazorla-Perfetti D. Revisión de los vectores de la enfermedad de Chagas en Venezuela (Hemiptera-Heteroptera, Reduviidae, Triatominae) Saber. 2016;28(3):387–470. [Google Scholar]
- 7.Méndez-Cardona S, Ortiz MI, Carrasquilla MC, Fuya P, Guhl F, González C. Altitudinal distribution and species richness of triatomines (Hemiptera Reduviidae) in Colombia. Parasit Vectors. 2022;15:450–450. doi: 10.1186/s13071-022-05574-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carcavallo RU, Tonn RJ, Carrasquero B. Distribución de Triatominos en Venezuela, (Hemiptera, Reduviidae) Actualización por entidades y zonas biogeográficas. Bol Dir Malariol San Amb. 1977;17(1):53–65. [Google Scholar]
- 9.Carcavallo RU, Tonn RJ, Jiménez JC. Notas sobre biología, ecología y distribución geográfica de Rhodnius neivai Lent, 1953 (Hemiptera, Reduviidae) Bol Dir Malariol San Amb. 1976;16(2):169–171. [Google Scholar]
- 10.Carcavallo RU, Rodriguez MEF, Salvatella R. Curto de Casas SI.Sherlock IS.Galvão C Carcavallo RU, Girón IG, Jurberg J. Atlas of Chagas' disease vectors in the Americas. Rio de Janeiro: Fiocruz; 1998. Habitats and related fauna; pp. 537–560. [Google Scholar]
- 11.Lent H, Wygodzinsky P. Revision of the Triatominae (Hemiptera, Reduviidae) and their significance as vectors of Chagas disease. Bull Am Mus Nat Hist. 1979;163:125–520. [Google Scholar]
- 12.Cabello DR. Resistance to starvation of Rhodnius neivai Lent, 1953 (Hemiptera Reduviidae: Triatominae) under experimental conditions. Mem Inst Oswaldo Cruz. 2001;96(4):587–591. doi: 10.1590/s0074-02762001000400023. [DOI] [PubMed] [Google Scholar]
- 13.Coura JR. The main sceneries of Chagas disease transmission The vectors, blood and oral transmissions - A comprehensive review. Mem Inst Oswaldo Cruz. 2015;110(3):277–282. doi: 10.1590/0074-0276140362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.López-García A, Gilabert JA. Oral transmission of Chagas disease from a one health approach a systematic review. Trop Med Int Health. 2023;28(9):689–698. doi: 10.1111/tmi.13915. [DOI] [PubMed] [Google Scholar]
- 15.Segura-Alba ML, Hernandez C, Guerra AP, Luna N, Cortes LJ, Acevedo CR. Acute Chagas disease outbreaks in Colombia in 2019. IJID Reg. 2024;12:100410–100410. doi: 10.1016/j.ijregi.2024.100410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher-Lartey SM, Caprarelli G. Application of GIS technology in public health successes and challenges. Parasitology. 2016;143(4):401–415. doi: 10.1017/S0031182015001869. [DOI] [PubMed] [Google Scholar]
- 17.Palaniyandi M. The role of remote sensing and GIS for spatial prediction of vector-borne diseases transmission a systematic review. J Vector Borne Dis. 2012;49(4):197–204. [PubMed] [Google Scholar]
- 18.Gurgel-Gonçalves R, Galvão C, Costa J, Peterson AT. Geographic distribution of Chagas disease vectors in Brazil based on ecological niche modeling. J Trop Med. 2012 doi: 10.1155/2012/705326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ceccarelli S, Balsalobre A, Susevich ML, Echeverria MG, Gorla DE, Marti GA. Modelling the potential geographic distribution of triatomines infected by Triatoma virus in the southern cone of South America. Parasit Vectors. 2015;8:153–153. doi: 10.1186/s13071-015-0761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrido R, Bacigalupo A, Peña-Gómez F, Bustamante RO, Cattan PE, Gorla DE. Potential impact of climate change on the geographical distribution of two wild vectors of Chagas disease in Chile Mepraia spinolai and Mepraia gajardoi. Parasit Vectors. 2019;12:478–478. doi: 10.1186/s13071-019-3744-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Paula AS, Barreto C, Telmo MCM, Diotaiuti L, Galvão C. Historical biogeography and the evolution of hematophagy in Rhodniini (Heteroptera Reduviidae: Triatominae) Front Ecol Evol. 2021;9:660151–660151. [Google Scholar]
- 22.Corrêa-do-Nascimento GS, Leite GR. Current and paleoclimate models for an Atlantic Forest kissing bug indicate broader distribution outside biome delimitations. Front Ecol Evol. 2023;10:1051454–1051454. [Google Scholar]
- 23.Wisz MS, Hijmans RJ, Li J, Peterson AT, Graham CH, Guisan A. NCEAS Predicting Species Distributions Working Group Effects of sample size on the performance of species distribution models. Divers Distrib. 2008;14:763–773. [Google Scholar]
- 24.Mateo RG, Felicísimo ÁM, Muñoz J. Effects of the number of presences on reliability and stability of MARS species distribution models the importance of regional niche variation and ecological heterogeneity. J Veg Sci. 2010;21:908–922. [Google Scholar]
- 25.Park DS, Davis CC. Implications and alternatives of assigning climate data to geographical centroids. J Biogeogr. 2017;44:2188–2198. [Google Scholar]
- 26.Cheng Y, Tjaden NB, Jaeschke A, Thomas SM, Beierkuhnlein C. Using centroids of spatial units in ecological niche modelling effects on model performance in the context of environmental data grain size. Glob Ecol Biogeogr. 2021;2:1–11. [Google Scholar]
- 27.Gorla DE, Noireau F. Geographic distribution of Triatominae vectors in America. Elsevier. 2017 [Google Scholar]
- 28.Guhl F, Aguilera G, Pinto N, Vergara D. Actualización de la distribución geográfica y ecoepidemiología de la fauna de triatominos (Reduviidae Triatominae) en Colombia. Biomedica. 2007;27(1):143–162. [PubMed] [Google Scholar]
- 29.Ceccarelli S, Balsalobre A, Medone P, Cano ME, Gurgel-Gonçalves R, Feliciangeli D. DataTri, a database of American triatomine species occurrence. Sci Data. 2018;5:180071–180071. doi: 10.1038/sdata.2018.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ceccarelli S, Balsalobre A, Vicente ME, Curtis-Robles R, Hamer SA, Ayala Landa JM, et al. American triatomine species occurrences: updates and novelties in the DataTri database. GigaByte. 2022 doi: 10.46471/gigabyte.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Diniz-Filho JA, Jardim L, Guedes JJM, Meyer L, Stropp J, Frateles LEF. Macroecological links between the Linnean, Wallacean, and Darwinian shortfalls. Front Biogeogr. 2023;15(2):e59566 [Google Scholar]
- 32.CEPAVE BibTri. 2023. https://bibtri.cepave.edu.ar/webbibtri.php
- 33.GBIF GBIF home page. 2023. https://www.gbif.org
- 34.UNOCHA The humanitarian data exchange. 2023. https://data.humdata.org/
- 35.ESRI ArcGIS. Redlands. 2019 https://www.esri.com/en-us/home?srsltid=AfmBOor9ayyXoE4fyB00vXVZlCqfAwjqN4ZA5gy1A2C5rLveAhV-nvWi [Google Scholar]
- 36.Morrone JJ, Escalante T, Rodríguez-Tapia G, Carmona A, Arana M, Mercado-Gómez JD. Biogeographic regionalization of the neotropical region new map and shapefile. An Acad Bras Cienc. 2022;94:e20211167. doi: 10.1590/0001-3765202220211167. [DOI] [PubMed] [Google Scholar]
- 37.Olson DM, Dinerstein E, Wikramanayake ED, Burgess ND, Powell GVN, Underwood EC. Terrestrial ecoregions of the world a new map of life on earth. BioScience. 2001;51(11):933–938. [Google Scholar]
- 38.Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW. Climatologies at high resolution for the earth's land surface areas. Sci Data. 2017;4:170122–170122. doi: 10.1038/sdata.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Karger DN, Conrad O, Böhner J, Kawohl T, Kreft H, Soria-Auza RW. Climatologies at high resolution for the earth's land surface areas. Sci Data. 2017;4:170122–170122. doi: 10.1038/sdata.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown JL, Hill DJ, Dolan AM, Carnaval AC, Haywood AM. PaleoClim, high spatial resolution paleoclimate surfaces for global land areas. Sci Data. 2018;5:180254–180254. doi: 10.1038/sdata.2018.254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Escobar LE, Lira-Noriega A, Medina-Vogel G, Peterson AT. Potential for spread of the white-nose fungus (Pseudogymnoascus destructans) in the Americas use of Maxent and NicheA to assure strict model transference. Geospat Health. 2014;9:221–221. doi: 10.4081/gh.2014.19. [DOI] [PubMed] [Google Scholar]
- 42.Samy AM, Elaagip AH, Kenawy MA, Ayres CFJ, Peterson AT, Soliman DE. Climate change influences on the global potential distribution of the mosquito Culex quinquefasciatus, vector of West Nile virus and lymphatic filariasis. PLoS One. 2016;11:e0163863. doi: 10.1371/journal.pone.0163863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raghavan RK, Barker SC, Cobos ME, Barker D, Teo EJM, Foley DH. Potential spatial distribution of the newly introduced long-horned tick, Haemaphysalis longicornis in North America. Sci Rep. 2019;9:498–498. doi: 10.1038/s41598-018-37205-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hosni EM, Nasser MG, Al-Ashaal SA, Rady MH, Kenawy MA. Modeling current and future global distribution of Chrysomya bezziana under changing climate. Sci Rep. 2020;10:4947–4947. doi: 10.1038/s41598-020-61962-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Booth TH. Checking bioclimatic variables that combine temperature and precipitation data before their use in species distribution models. Austral Ecol. 2022;47:1506–1514. [Google Scholar]
- 46.Phillips SJ, Dudík M, Schapire RE. Maxent software for modeling species niches and distributions (Version 3, 4.4) 2022. http://biodiversityinformatics.amnh.org/open_source/maxent/
- 47.R Core Team R: a language and environment for statistical computing. R Foundation for Statistical Computing. 2022 https://www.r-project.org/ [Google Scholar]
- 48.Bender A, Python A, Lindsay SW, Golding N, Moyes CL. Modelling geospatial distributions of the triatomine vectors of Trypanosoma cruzi in Latin America. PLoS Negl Trop Dis. 2020;14(8):e0008411. doi: 10.1371/journal.pntd.0008411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cobos ME, Peterson AT, Barve N, Osorio-Olvera L. kuenm: an R package for detailed development of ecological niche models using Maxent. PeerJ. 2019 doi: 10.7717/peerj.6281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dormann CF, Elith J, Bacher S, Buchmann C, Carl G, Carré G. Collinearity a review of methods to deal with it and a simulation study evaluating their performance. Ecography. 2013;36:27–46. [Google Scholar]
- 51.Altamiranda-Saavedra M, Osorio-Olvera L, Yáñez-Arenas C, Marín-Ortiz JC, Parra-Henao G. Geographic abundance patterns explained by niche centrality hypothesis in two Chagas disease vectors in Latin America. PLoS One. 2020;15:e0241710. doi: 10.1371/journal.pone.0241710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown JL. SDMtoolbox a python-based GIS toolkit for landscape genetic, biogeographic and species distribution model analyses. Methods Ecol Evol. 2014;5:694–700. doi: 10.7717/peerj.4095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobos ME, Peterson AT, Osorio-Olvera L, Jiménez-García D. An exhaustive analysis of heuristic methods for variable selection in ecological niche modeling and species distribution modeling Ecol. Inform. 2019;3:100983–100983. [Google Scholar]
- 54.Peterson AT, Papes M, Soberón J. Rethinking receiver operating characteristic analysis applications in ecological niche modeling. Ecol Modell. 2008;213:63–72. [Google Scholar]
- 55.Lobo JM, Jiménez-Valverde A, Real R. AUC a misleading measure of the performance of predictive distribution models. Glob Ecol Biogeogr. 2008;17(2):145–151. [Google Scholar]
- 56.Qiao H, Soberón J, Peterson AT. No silver bullets in correlative ecological niche modelling insights from testing among many potential algorithms for niche estimation. Methods Ecol Evol. 2015;6:1126–1136. [Google Scholar]
- 57.De Marco PJ, Nóbrega CC. Evaluating collinearity effects on species distribution models an approach based on virtual species simulation. PLoS One. 2018;13:e0202403. doi: 10.1371/journal.pone.0202403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Andrade AFA, Velazco SJE, De Marco PJ. ENMTML an R package for a straightforward construction of complex ecological niche models. Environ Modell Softw. 2020;125:104615–104615. [Google Scholar]
- 59.DER - División de Endemias Rurales Campaña contra la enfermedad de Chagas. Revista Venezolana de Sanidad y Asistencia Social. 1965;31(1):113–154. [PubMed] [Google Scholar]
- 60.Veliz O, Morillo N, Torres R, Bonfante R. Rhodnius neivai naturalmente infectado con Trypanosoma cruzi en la ciudad de Barquisimeto, Estado Lara, Venezuela. Acta Méd Venez. 1972;19:392–393. [Google Scholar]
- 61.Otero MA, Jiménez JC, Carcavallo RU, Ortega R, Tonn RJ. Actualización de la distribución geográfica de Triatominae (Hemiptera, Reduviidae) en Venezuela. Boletín de la Dirección de Malariología y Saneamiento Ambiental. 1975;15(5):217–230. [Google Scholar]
- 62.Otero MA, Giménez JC, Ortega R, Carcavallo R, Tonn R. New records of geografical distribution of Triatominae in Venezuela. WHO/VBC. 1975 [Google Scholar]
- 63.de Olaria TR. Nota sobre la presencia de Rhodnius neivai Lent, 1953 (Hemiptera, Reduviidae-Triatominae) en el municipio Chinquinquira Distrito Maracaibo, estado Zulia - Venezuela. Kasmera. 1985;13:1–4. [Google Scholar]
- 64.Gonçalves TCM, Almeida MD, Jurberg J, Lent H. Lista dos exemplares-tipos de triatomíneos depositados na coleção entomológica do Instituto Oswaldo Cruz, Rio de Janeiro (Hemiptera Reduviidae) Mem Inst Oswaldo Cruz. 1993;88(2):327–333. [Google Scholar]
- 65.Körner C. Mountain biodiversity, its causes and function. Ambio. 2004;13:11–17. [PubMed] [Google Scholar]
- 66.Lent H. Um novo hemiptero hematófago da Venezuela (Reduviidae, Triatominae) Rev Bras Biol. 1953;13(2):169–172. [Google Scholar]
- 67.Cova-Garcia P, Suarez M. Estudio de los Triatominos en Venezuela Ministerio de Sanidad y Asistencia Social, Caracas, Venezuela. Publicaciones de la División de Malariología. 1959;11:209–209. [Google Scholar]
- 68.Lent H, Jurberg J. O género Rhodnius stal, 1859, com um estudo sobre a genitália das espécies (Hemiptera, Reduviidae, Triatominae) Rev Bras Biol. 1969;29(4):487–560. [Google Scholar]
- 69.Gamboa CJ. Distribución geográfica y prevalencia de la población de Triatominos en Venezuela. Maracay: Report to the CDVRU Planning Meeting; 1973. [Google Scholar]
- 70.Harry M, Roose CL, Vautrin D, Noireau F, Romaña CA, Solignac M. Microsatellite markers from the Chagas disease vector, Rhodnius prolixus (Hemiptera, Reduviidae), and their applicability to Rhodnius species. Infect Genet Evol. 2008;8(3):381–385. doi: 10.1016/j.meegid.2008.01.006. [DOI] [PubMed] [Google Scholar]
- 71.Pita S, Panzera F, Ferrandis I, Galvão C, Gómez-Palacio A, Panzera Y. Chromosomal divergence and evolutionary inferences of Rhodniini based on the chromosomal location of ribosomal genes. Mem Inst Oswaldo Cruz. 2013;108(3):376–382. doi: 10.1590/S0074-02762013000300017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.D'Alessandro A.Barreto P Carcavallo RW, Rabinovich JE, Tonn RJ. Factores biológicos y ecológicos en la enfermedad de Chagas. Buenos Aires: ECO/SNCH; 1985. Colombia; pp. 377–400. [Google Scholar]
- 73.Morales A, Ferro C. Isaza de Rodríguez C.Cura E Encuesta sobre artropodos de interés médico en La Guajira, Colombia, Suramérica. Biomedica. 1987;7(3-4):89–94. [Google Scholar]
- 74.Perrigo A, Hoorn C, Antonelli A. Why mountains matter for biodiversity. J Biogeogr. 2020;47:315–325. [Google Scholar]
- 75.Rodriguero MS, Gorla DE. Latitudinal gradient in species richness of the New World Triatominae (Reduviidae) Glob Ecol Biogeogr. 2004;13:75–84. [Google Scholar]
- 76.Diniz-Filho JAF, Ceccarelli S, Hasperué W, Rabinovich J. Geographical patterns of Triatominae (Heteroptera Reduviidae) richness and distribution in the Western Hemisphere. Insect Conserv Divers. 2013;6:704–714. [Google Scholar]
- 77.Parra-Henao G, Quirós-Gómez O, Jaramillo-O N, Cardona ÁS. Environmental determinants of the distribution of Chagas disease vector Triatoma dimidiata in Colombia. Am J Trop Med Hyg. 2016;94(4):767–774. doi: 10.4269/ajtmh.15-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Parra-Henao G, Suárez-Escudero LC, González-Caro S. Potential distribution of Chagas disease vectors (Hemiptera, Reduviidae, Triatominae) in Colombia, based on ecological niche modeling. J Trop Med. 2016;2016:1439090–1439090. doi: 10.1155/2016/1439090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ceccarelli S, Rabinovich JE. Global climate change effects on Venezuela's vulnerability to Chagas disease is linked to the geographic distribution of five triatomine species. J Med Entomol. 2015;52(6):1333–1343. doi: 10.1093/jme/tjv119. [DOI] [PubMed] [Google Scholar]
- 80.Medone P, Ceccarelli S, Parham PE, Figuera A, Rabinovich JE. The impact of climate change on the geographical distribution of two vectors of Chagas disease implications for the force of infection. Philos Trans R Soc Lond B Biol Sci. 2015;370(1665):20130560–20130560. doi: 10.1098/rstb.2013.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Barve N, Barve V, Jiménez-Valverde A, Lira-Noriega A, Maher SP, Peterson AT. The crucial role of the accessible area in ecological niche modeling and species distribution modeling. Ecol Modell. 2011;222(11):1810–1819. [Google Scholar]
- 82.De La Vega GJ.Schilman PE Ecological and physiological thermal niches to understand distribution of Chagas disease vectors in Latin America. Med Vet Entomol. 2018;32(1):1–13. doi: 10.1111/mve.12262. [DOI] [PubMed] [Google Scholar]
- 83.Sánchez-Cuervo AM, Aide TM, Clark ML, Etter A. Land cover change in Colombia surprising forest recovery trends between 2001 and 2010. PLoS One. 2012;7(8):e43943. doi: 10.1371/journal.pone.0043943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Justi SA, Galvão C, Schrago CG. Geological changes of the Americas and their influence on the diversification of the neotropical kissing bugs (Hemiptera Reduviidae: Triatominae) PLoS Negl Trop Dis. 2016;10:e0004527. doi: 10.1371/journal.pntd.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Abad-Franch F, Monteiro FA, Jaramillo ON, Gurgel-Gonçalves R, Dias FB, Diotaiuti L. Ecology, evolution, and the long-term surveillance of vector-borne Chagas disease a multi-scale appraisal of the tribe Rhodniini (Triatominae) Acta Trop. 2009;110:159–177. doi: 10.1016/j.actatropica.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 86.Ibarra-Cerdeña CN, Zaldívar-Riverón A, Peterson AT, Sánchez-Cordero V, Ramsey JM. Phylogeny and niche conservatism in North and Central American triatomine bugs (Hemiptera Reduviidae: Triatominae), vectors of Chagas' disease. PLoS Negl Trop Dis. 2014;8(10):e3266. doi: 10.1371/journal.pntd.0003266. [DOI] [PMC free article] [PubMed] [Google Scholar]


