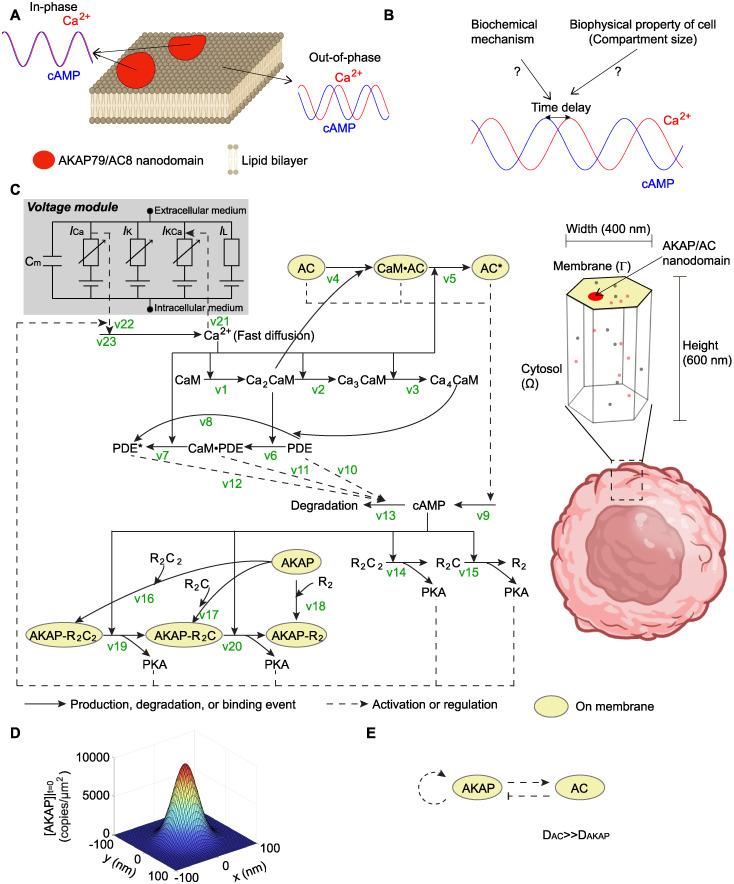
Fig 1. The phase regulation of Ca2+-cAMP oscillation and corresponding mathematical modeling.
(A) Schematic showing out-of-phase Ca2+-cAMP oscillation outside the nanodomain and in-phase behavior when localized to AKAP/AC nanodomains. This schematic is designed based on experiments in [32] (created with BioRender.com). (B) In this work, we aim to explore the biochemical mechanism of the phase difference (or time delay) between Ca2+ and cAMP oscillation and study the possible contributing factors. (C) Mathematical modeling of the AKAP-Ca2+-cAMP circuit. On the left-hand side, the diagram of the signaling pathway is shown; the solid arrow indicates production, degradation, or binding events, and the dashed arrow indicates the regulation effect that usually does not consume the reactants. The voltage module (highlighted in gray) includes a capacitor with the membrane capacitance Cm and four ion channels: Ca2+ channel, K+ channel, Ca2+ gated K+ channel, and leak channel. Currents for ion channels are represented by ICa, IK, IKCa, and IL, where the subscript indicates the specific ion channel. On the right-hand side, the simulation domain is a hexagonal prism, which is only a small compartment of one cell (created with BioRender.com). In this compartment, the top surface (yellow area) denotes the cell membrane; the AKAP/AC nanodomain (the large patch in red) is located on the cell membrane; the volume under the top surface is cytosol. Molecules (dots in gray and orange) can diffuse in the cytosol or on the membrane depending on the location of the molecule. (D) A single AKAP/AC nanodomain was modeled using a Gaussian distribution. (E) Interactions between AC and AKAP that can generate a Turing pattern.