Abstract
BACKGROUND
Intra-operative urine output (UO) has been shown to predict postoperative acute kidney injury (AKI) in adults; however, its significance in children undergoing cardiac surgery remains unknown.
OBJECTIVE
To explore the association between intra-operative UO and postoperative AKI in children with congenital heart disease.
DESIGN
A retrospective observational study.
SETTING
A tertiary hospital.
PATIENTS
Children aged >28 days and <6 years who underwent cardiac surgery at Fuwai Hospital from 1 April 2022 to 30 August 2022.
MAIN OUTCOME MEASURES
AKI was identified by the highest serum creatinine value within postoperative 7 days using Kidney Disease Improving Global Outcomes (KDIGO) criteria.
RESULTS
In total, 1184 children were included. The incidence of AKI was 23.1% (273/1184), of which 17.7% (209/1184) were stage 1, 4.2% (50/1184) were stage 2, and others were stage 3 (1.2%, 14/1184). Intra-operative UO was calculated by dividing the total intra-operative urine volume by the duration of surgery and the actual body weight measured before surgery. There was no significant difference in median [IQR] intra-operative UO between the AKI and non-AKI groups (2.6 [1.4 to 5.4] and 2.7 [1.4 to 4.9], respectively, P = 0.791), and multivariate logistic regression analyses showed that intra-operative UO was not associated with postoperative AKI [adjusted odds ratio (OR) 0.971; 95% confidence interval (CI), 0.930 to 1.014; P = 0.182]. Regarding the clinical importance of severe forms of AKI, we further explored the association between intra-operative UO and postoperative moderate-to-severe AKI (adjusted OR 0.914; 95% CI, 0.838 to 0.998; P = 0.046).
CONCLUSIONS
Intra-operative UO was not associated with postoperative AKI during paediatric cardiac surgery. However, we found a significant association between UO and postoperative moderate-to-severe AKI. This suggests that reductions in intra-operative urine output below a specific threshold may be associated with postoperative renal dysfunction.
TRIAL REGISTRATION
Clinicaltrials.gov identifier: NCT05489263.
KEY POINTS
The relationship between intra-operative urine output (UO) and postoperative acute kidney injury (AKI) in children with congenital heart disease remains unclear.
This study demonstrated a significant association between intra-operative UO and moderate-to-severe AKI in children following surgery for congenital heart disease.
Monitoring intra-operative UO may prevent moderate-to-severe AKI if kidney protective measures are followed.
Introduction
Acute kidney injury (AKI) is a common complication after paediatric cardiac surgery and has a significant association with worse short and long-term outcomes, such as increased in-hospital mortality, prolonged hospital and intensive care unit stay, and chronic kidney disease.1,2 Owing to different types of surgery and varied diagnostic criteria, the reported incidence of postoperative AKI in paediatric cardiac surgery ranges from 9% to 50%.3,4
Serum creatinine (SCr) and urine output (UO) are representative indicators both for the diagnosis and for assessment of the treatment effect of AKI.5 However, isolated and notable SCr changes often lag slightly behind renal impairment, which might delay both the detection and therapy of AKI. Therefore, timely identification of reliable markers is essential for preventing postoperative AKI, and this has become a popular topic in recent years.6 As a crucial indicator of renal perfusion, reduced UO is believed to be attributed to systemic hypovolaemia or sustained hypoperfusion of the kidneys. Intra-operative UO predicts postoperative AKI in adults undergoing cardiac7 and noncardiac procedures.8,9
Congenital heart disease is a major cause of hospital-acquired AKI and accounts for 19% of cases approximately.10 Following cardiopulmonary bypass (CPB) surgery for congenital heart disease, children often experience abnormal blood flow patterns, renal ischaemia-reperfusion injury, and systemic inflammatory response, both due to the primary cardiac defect and the surgical intervention, resulting in a high risk of new-onset postoperative AKI.11,12 Evidence from previous studies in children on the association between intra-operative UO and postoperative AKI under conditions of disturbed haemodynamic status remains unclear. Therefore, we hypothesised that intra-operative UO would have a significant association with postoperative renal injury in children undergoing congenital heart surgery. This study aimed to explore the association between intra-operative UO and postoperative AKI in children after surgery for congenital cardiac defects.
Methods
Ethics statements
Ethical approval for this study was provided by the Ethical Committee of Fuwai Hospital, Beijing, China on 3 March 2022 (ethical approval number: 2021-1607). The data used in this retrospective study were obtained from a trial registered with Clinicaltrials.gov (Clinicaltrials.gov identifier: NCT05489263; A Predictive Score System for AKI Following Paediatric Cardiac Surgery), and written informed consent was obtained from all participants and/or their legal representatives before enrolment. This study followed the Strengthening the Reporting of Observational Studies in Epidemiology reporting guideline for observational studies.
Study design and population
This retrospective observational study used a prospectively collected peri-operative clinical dataset. We included infants and children aged >28 days and <6 years who underwent surgical repair of congenital cardiac disease with CPB between 1 April 2022 and 30 August 2022 at Fuwai Hospital. We excluded patients if they had chronic renal disease, defined as preoperative estimated glomerular filtration rate of <60 ml min–1 1.73 m–2 or requiring dialysis;13 had unavailable preoperative SCr and postoperative SCr values within postoperative day (POD) 7; had off-pump surgery; did not have intra-operative UO records; and were re-explored within 7 days after surgery. For patients who underwent multiple admissions for cardiac surgery during the study period, only the first admission was considered.
Data collection
All data were obtained from a dataset in which peri-operative clinical data were prospectively collected from the electronic medical records. Personal and clinical characteristics, laboratory biomarkers, intra-operative information, and postoperative data were recorded in real-time by caregivers.
Personal characteristics included age at surgery, sex, weight, and gestational age. Preoperative variables that may be associated with postoperative AKI were selected according to previous reports and clinical knowledge,14 including the Risk Stratification for Congenital Heart Surgery (RACHS-1) score, previous cardiac surgery history, left ventricular ejection fraction, laboratory test results [haemoglobin (Hb) concentration and SCr], mean arterial pressure (MAP), and diuretic administration. Surgical variables included CPB duration, aortic cross-clamping (ACC) duration, lowest core temperature during CPB, blood transfusion, intravenous fluid administration, furosemide use, furosemide dose, blood loss, UO, lowest Hb level during surgery, Hb level after CPB, modified ultrafiltration and vasoactive-inotropic score (VIS).
Intra-operative UO was routinely recorded by the anaesthetic team. The average intra-operative UO was retrospectively calculated as the UO per body weight of the patient per hour (ml kg−1 h−1), and was arrived at by dividing the total intra-operative urine volume by the duration of surgery and the actual body weight measured before surgery. Additionally, the intravenous fluid administration volume and blood loss were adjusted for body weight. Duration of surgery was from anaesthesia induction to the end of wound closure, and SCr concentrations were measured daily during the intensive care unit (ICU) stay.
Outcomes
The highest SCr value within POD 7 was used with the KDIGO criteria to identify AKI (Table 1).15 For all analyses, we used moderate-to-severe AKI, defined as stages 2 to 3. The most recent preoperative SCr value was used as the baseline value.
Table 1.
Acute kidney injury definition according to Kidney Disease Improving Global Outcomes
| Stage | Serum creatinine | Urine output |
| 1 | 1.5 to 1.9 times baseline or ≥0.3 mg dl−1 (≥26.5 μmol l−1) increase | <0.5 ml kg−1h−1 for 6 to 12 h |
| 2 | 2.0 to 2.9 times baseline | <0.5 ml kg−1−h−1 for ≥12 h |
| 3 | 3.0 times baseline or increase in serum creatinine to ≥4.0 mg dl−1 (≥353.6 μmol l−1) or a initiation of renal replacement therapy or in patients <18 years a decrease in eGFR to <35 ml min−1 1.73−m−2 | <0.3 ml kg−1−h−1 for ≥24 h or anuria for ≥12 h |
Statistical analysis
We explored the relationship between intra-operative UO and any AKI (stages 1 to 3) using multivariate logistic regression analysis, where missing values were imputed using median or mode, as appropriate. Continuous variables are presented as mean ± SD or median [IQR]. Categorical variables are computed as frequency and proportion. Continuous variables were compared using the Student's t-test, Mann–Whitney U-test, or Kruskal–Wallis test, and categorical variables were compared using the χ2 or Fisher exact test. Variables with a P-value of <0.2 in the univariate analysis or clinically relevant variables were included in an enter selection multivariate logistic regression model with intra-operative UO. We included only one factor if two factors were correlated (i.e. variance inflation factor of >5) to avoid collinearity.16 The effect size was quantified using the adjusted OR and 95% CI.
Given that the severe form of AKI has a significant association with morbidity and mortality17 and that peri-operative mild AKI may not be of clinical importance,18 we further explored the potential association between intra-operative UO and severe postoperative forms of AKI in posthoc analysis. Owing to the low incidence of moderate and severe AKI in this cohort, we grouped moderate-to-severe AKI (stages 2 and 3) together for multivariate logistic regression analyses and adjusted for both statistically and clinically relevant variables. Patients with non-AKI and mild AKI were also compared to determine the association between UO and mild postoperative AKI.
We used SPSS software (version 25.0; SPSS, Chicago, IL, USA) and R software (version 4.2.0; R Foundation for Statistical Computing, Vienna, Austria) to perform statistical analyses. A P-value of <0.05 was considered statistically significant.
Results
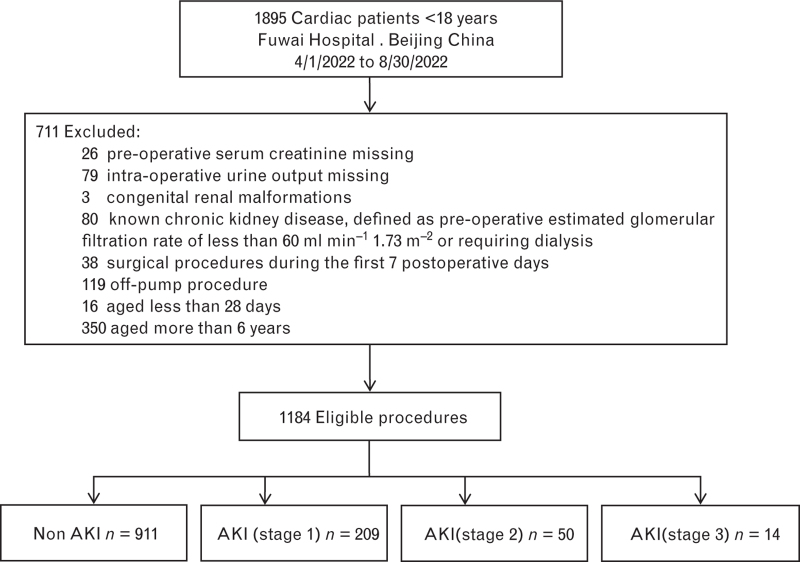
Of the 1895 children who underwent congenital heart surgery during the study period in our hospital, 1184 were included in the final analysis (Fig. 1). The baseline, preoperative, intra-operative, and postoperative characteristics are summarised in Table 2 for the whole group, and for patients who did and did not develop AKI. The overall median [IQR] age was 22.3 [8.4 to 43.5] months, and the intra-operative UO was 2.7 [1.4 to 5.0] ml kg−1 h−1. Approximately 42% of children were designated as having a RACHS-1 score of ≥3. The incidence of postoperative AKI on POD 7 was 23.1% (273/1184), and moderate-to-severe AKI occurred in 64 patients (5.4%) (Table 3).
Fig. 1.
Flow chart. AKI, acute kidney injury.
Table 2.
Baseline and peri-operative characteristics of patients with and without acute kidney injury
| All (n = 1184) | Non-AKI (n = 911) | AKI (n = 273) | P-value | |
| Baseline characteristics | ||||
| Female | 569 (48.1) | 449 (49.3) | 120 (44.0) | 0.14 |
| Age at surgery (months) | 22.3 [8.4 to 43.5] | 29.2 [10.7 to 47.8] | 9.2 [4.7 to 24.9] | <0.001 |
| Weight (kg) | 11.7 ± 5.0 | 12.5 ± 5.0 | 9.1 ± 4.1 | <0.001 |
| Gestational age (weeks) | 39 [38 to 40] | 39 [38 to 40] | 39 [37 to 40] | 0.059 |
| Preoperative data | ||||
| Previous cardiac surgery | 58 (4.9) | 46 (5.0) | 12 (4.4) | 0.78 |
| RACHS-1 ≥3 | 499 (42.1) | 367 (40.2) | 132 (48.3) | 0.018 |
| LVEF (%) | 69 [65 to 73] | 69 [65 to 73] | 69 [65 to 72] | 0.319 |
| MAP (mmHg) | 67 [63 to 70] | 68 [64 to 71] | 65 [63 to 69] | <0.001 |
| Serum creatinine (μmol l−1) | 28 [22 to 34] | 29 [23 to 36] | 23 [19 to 29] | <0.001 |
| Haemoglobin (g dl−1) | 12 [11.2 to 12.8] | 12.2 [11.4 to 12.9] | 11.5 [10.6 to 12.3] | <0.001 |
| Use of diuretics | 93 (7.9) | 63 (6.9) | 30 (11.0) | 0.039 |
| Intra-operative data | ||||
| CPB duration (min) | 71 [48 to 99] | 68 [47 to 94] | 80 [55 to 122] | <0.001 |
| ACC duration (min) | 44 [27 to 67] | 42 [26 to 63] | 49 [32 to 77] | <0.001 |
| Blood transfusion | 78 (6.6) | 52 (5.7) | 26 (9.5) | 0.037 |
| Fluid administration (ml kg−1) | 5.2 [3.5 to7.9] | 4.7 [3.3 to 7.4] | 6.9 [4.6 to 9.8] | <0.001 |
| Urine output (ml kg−1h−1) | 2.7 [1.4 to 5.0] | 2.7 [1.4 to 4.9] | 2.6 [1.4 to 5.4] | 0.791 |
| Use of furosemide | 923 (78.0) | 704 (77.3) | 219 (80.2) | 0.344 |
| Furosemide dose (mg kg−1) | 0.4 [0.2 to 0.6] | 0.4 [0.2 to 0.5] | 0.5 [0.3 to 0.8] | <0.001 |
| Blood loss (ml kg−1) | 1.3 [1.0 to 1.8] | 1.2 [0.9 to 1.7] | 1.5 [1.2 to 2.1] | <0.001 |
| Vasoactive-inotropic score | 12 [6 to13] | 12 [6 to 12] | 12 [11 to 16] | <0.001 |
| Haemoglobin after CPB (g dl−1) | 8.8 [8.2 to 9.7] | 8.8 [8.2 to 9.7] | 8.9 [8.1 to 9.6] | 0.94 |
| Lowest Haemoglobin (g dl−1) | 9.3 [8.7 to 9.6] | 9.3 [8.7 to 9.7] | 9.3 [8.7 to 9.6] | 0.926 |
| Lowest core temperature during CPB (°C) | 32.9 [31.4 to 33.9] | 33.1 [31.7 to34.2] | 32.0 [30.8 to 33.2] | <0.001 |
| Modified ultrafiltration | 990 (83.6) | 737 (80.9) | 253 (92.7) | <0.001 |
| Postoperative outcomes | ||||
| Length of MV (h) | 3 [0 to 6] | 3 [0 to 5] | 4 [0 to 19] | <0.001 |
| Length of ICU stay (days) | 2 [1 to 4] | 2 [1 to 3] | 3 [1 to 5] | <0.001 |
| Length of hospital stay (days) | 6 [5 to 9] | 6 [5 to 8] | 7 [5 to 10] | <0.001 |
Data are given as n (%), median [IQR] and mean ± SD as appropriate.
ACC, aortic cross-clamp; CPB, cardiopulmonary bypass; ICU, intensive care unit; LVEF, left ventricular ejection fractions; MAP, mean arterial pressure; MV, mechanical ventilation; RACHS-1, risk adjustment in congenital heart surgery-1 method.
Table 3.
Baseline and peri-operative characteristics of patients with and without different stages of acute kidney injury
| Non-AKI (n = 911) | Mild AKI (n = 209) | Moderate to severe AKI (n = 64) | P-value | |
| Baseline characteristics | ||||
| Female | 449 (49.3) | 94 (45.0) | 26 (40.6) | 0.251 |
| Age at surgery (months) | 29.2 [10.7 to 47.8] | 11.2 [5.6 to 27.5] | 5.7 [3.8 to 12.3] | <0.001 |
| Weight (kg) | 12.5 ± 5.0 | 9.7 ± 4.2 | 7.3 ± 3.0 | <0.001 |
| Gestational age (weeks) | 39 [38 to 40] | 39 [38 to 40] | 38 [37 to 40] | 0.140 |
| Preoperative data | ||||
| Previous cardiac surgery | 46 (5.0) | 10 (4.8) | 2 (3.1) | 0.786 |
| RACHS-1> = 3 | 367 (40.2) | 98 (46.9) | 34 (53.1) | 0.041 |
| LVEF (%) | 69 [65 to 73] | 68 [65 to 72] | 70 [65 to 72] | 0.572 |
| MAP (mmHg) | 68 [64 to 71] | 66 [63 to 70] | 64 [62 to 67] | <0.001 |
| Serum creatinine (μmol l−1) | 29 [23 to 36] | 24 [19 to 30] | 20 [17 to 22] | <0.001 |
| Haemoglobin (g dl−1) | 12.2 [11.4 to 12.9] | 11.6 [10.8 to 12.3] | 11.2 [10.2 to 12.3] | <0.001 |
| Use of diuretics | 63 (6.9) | 21 (10.0) | 9 (14.1) | 0.052 |
| Intra-operative data | ||||
| CPB duration (min) | 68 [47 to 94] | 76 [53 to 122] | 103 [69 to 145] | <0.001 |
| ACC duration (min) | 42 [26 to 63] | 47 [29 to 70] | 61 [45 to 95] | <0.001 |
| Blood transfusion | 52 (5.7) | 17 (8.1) | 9 (14.1) | 0.021 |
| Fluid administration (ml kg−1) | 4.7 [3.3 to 7.4] | 6.6 [4.3 to 8.9] | 8.2 [5.6 to 10.9] | <0.001 |
| Urine output (ml kg−1h−1) | 2.7 [1.4 to 4.9] | 2.7 [1.3 to 5.2] | 2.4 [1.5 to 5.5] | 0.964 |
| Use of furosemide | 704 (77.3) | 165 (78.9) | 54 (84.4) | 0.387 |
| Furosemide dose (mg kg−1) | 0.4 [0.2 to 0.5] | 0.5 [0.3 to 0.7] | 0.7 [0.4 to 0.9] | <0.001 |
| Blood loss (ml kg-1) | 1.2 [0.9 to 1.7] | 1.4 [1.1 to 1.9] | 2.1 [1.5 to 2.6] | <0.001 |
| Vasoactive-inotropic score | 12 [6 to 12] | 12 [10 to 16] | 12 [12 to 16] | <0.001 |
| Haemoglobin after CPB (g dl−1) | 8.8 [8.2 to 9.7] | 9.0 [8.1 to 9.7] | 8.8 [7.9 to 9.5] | 0.324 |
| Lowest haemoglobin (g dl−1) | 9.3 [8.7 to 9.7] | 9.3 [8.7 to 9.6] | 9.2 [8.8 to 9.5] | 0.005 |
| Lowest core temperature during CPB (°C) | 33.1[31.7 to34.2] | 32.0 [30.9 to 33.4] | 31.2 [30.2 to 32.1] | <0.001 |
| Modified ultrafiltration | 737 (80.9) | 193 (92.3) | 60 (93.8) | <0.001 |
| Postoperative outcomes | ||||
| Length of MV (h) | 3 [0 to 5] | 4 [0 to 12] | 7 [0 to 65] | <0.001 |
| Length of ICU stay (days) | 2 [1 to 3] | 2 [1 to 4] | 4 [2 to 11] | <0.001 |
| Length of hospital stay (days) | 6 [5 to 8] | 7 [5 to 9] | 9 [6 to 13] | <0.001 |
Data are given as n (%), median [IQR] or mean ± SD as appropriate.
ACC, aortic cross-clamp; CPB, cardiopulmonary bypass; ICU, intensive care unit; LVEF, left ventricular ejection fractions; MAP, mean arterial pressure; MV, mechanical ventilation; RACHS-1, risk adjustment in congenital heart surgery-1 method.
In univariate analysis, the AKI group was significantly younger; had lower preoperative MAP, SCr, and Hb values; and a higher proportion of diuretic use than the non-AKI group. Additionally, compared with the non-AKI group, the AKI group was more likely to have complex cardiac procedures (RACHS-1 score of ≥3), longer CPB and aortic cross-clamp durations, more intra-operative blood loss, and a higher VIS. The AKI group was more likely to undergo modified ultrafiltration and blood transfusion, have a higher volume of fluid administration per body weight, and receive more furosemide intra-operatively than the non-AKI group (all P < 0.05, Table 2). There was no significant statistical difference in intra-operative UO between the AKI and non-AKI groups (2.6 [1.4 to 5.4] and 2.7 [1.4 to 4.9], respectively; P = 0.791). To account for baseline and peri-operative differences, multivariate logistic regression analysis was used to adjust for potential confounders with a P-value of <0.2 and clinically relevant variables in univariate analysis including sex, age, gestation weeks, baseline serum creatinine, RACHS-1 ≥3, MAP, preoperative diuretic use, cardiac surgery history, haemoglobin, aortic cross-clamp duration, intra-operative furosemide dose, blood loss, fluid administration, blood transfusion, the lowest core temperature during CPB, modified ultrafiltration and vasoactive-inotropic score. We still did not find a statistically significant association between intra-operative UO and postoperative AKI stage (adjusted OR, 0.971; 95% CI, 0.930 to 1.014; P = 0.182) (Table 4).
Table 4.
Association between intra-operative urine output and postoperative acute kidney injury
| Adjusted OR | 95% CI | P-value | |
| Any stage AKI (non vs. Stage 1.2.3) | 0.971 | 0.930 to 1.014 | 0.182 |
| Mild AKI (non vs. Stage 1) | 0.986 | 0.942 to 1.033 | 0.551 |
| Moderate to severe AKI (non vs. Stage 2.3) | 0.914 | 0.838 to 0.998 | 0.046 |
The estimated ORs were obtained from multivariate logistic regression models.
AKI, acute kidney injury; CI, confidence interval; OR, odds ratio.
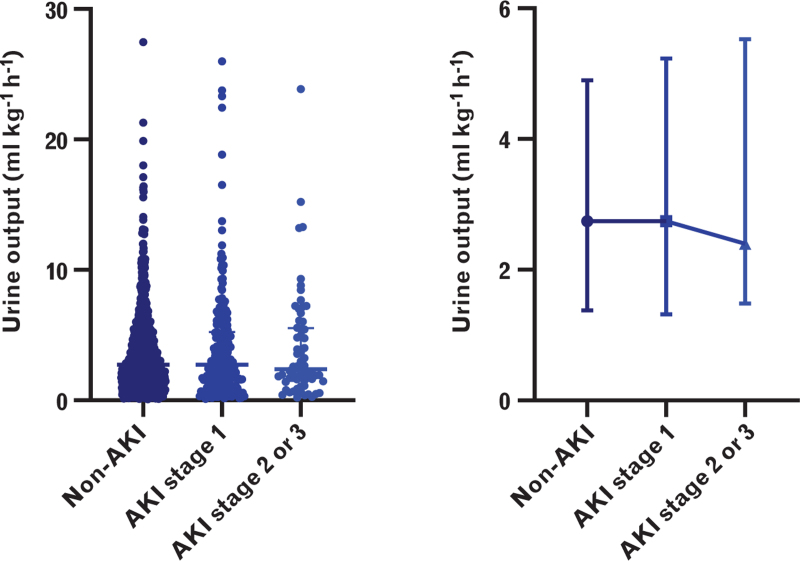
The distribution of intra-operative UO according to AKI severity is shown in Fig. 2. It showed a slightly reducing trend that was somewhat consistent with the UO diagnostic criteria of AKI: less UO is associated with worse kidney function.15 In the posthoc analysis, we did not find a significant association between UO and mild AKI (adjusted OR, 0.986; 95% CI, 0.942 to 1.033; P = 0.551). We further examined the relationship between intra-operative UO and the severe form of postoperative AKI in patients without AKI and those with moderate-to-severe AKI. Baseline and peri-operative variables are presented in Table 3. The median intra-operative UO of patients with moderate-to-severe AKI was less than that in those without AKI (2.4 [1.5 to 5.5] and 2.7 [1.4 to 4.9] ml kg−1 h−1, respectively). After adjusting for both statistically and clinically relevant factors including age, baseline serum creatinine, RACHS-1 ≥3, MAP, aortic cross-clamp duration, intra-operative furosemide dose, blood loss, fluid administration, blood transfusion, the lowest core temperature during CPB, vasoactive-inotropic score and modified ultrafiltration, we found a significant association between intra-operative UO and postoperative moderate-to-severe AKI (adjusted OR, 0.914; 95% CI, 0.838 to 0.998; P = 0.046). The results of the multivariate logistic regression analysis are presented in Table 4.
Fig. 2.
Distribution of intra-operative UO according to the AKI stages; AKI, acute kidney injury; UO, urine output.
Discussion
In this study, we evaluated the relationship between intra-operative UO and postoperative AKI in children who underwent surgery for congenital cardiac disorders. The overall incidence of AKI was 23.1%. Consistent with previous studies, our study showed that patients with AKI had worse clinical outcomes, longer mechanical ventilation times, longer ICU stays, and longer hospital stays than those without AKI. The main finding was that intra-operative UO was not associated with postoperative AKI. In the posthoc analyses of the non-AKI and moderate-to-severe AKI groups, we found a significant association between intra-operative UO and stage 2/3 AKI.
Since no widely available therapeutic strategies have been identified for patients with AKI, early recognition of the potential risk factors for postoperative AKI is necessary. As an early marker of renal injury, UO has been included in all the AKI diagnostic criteria.19 Studies concerning intra-operative oliguria and postoperative AKI in cardiac surgery are rare, especially in children. We explored this relationship in children undergoing congenital heart surgery, and the results of our study were partly consistent with the studies above. The intra-operative UO in the moderate-to-severe AKI group was lower than that in the non-AKI group, and there was a significant relationship between lower intra-operative UO and postoperative moderate-to-severe AKI (Table 4).
During cardiac surgery, a reduced intra-operative UO is probably an early indication of compromised renal function. Non-pulsatile flow during cardiopulmonary bypass, systemic inflammation, and release of free Hb may induce microcirculatory dysfunction and endothelial dysfunction with capillary leakage and impaired glomerular filtration rate.20 Additionally, reduced intravascular flow or sustained general hypoperfusion may cause a reduction in renal perfusion and result in decreased filtration load, potentially contributing to postoperative renal impairment and other adverse outcomes.21–23 Hence, patients with decreased intra-operative UO may be more likely to progress to postoperative AKI than those without.
We did not find an association between intra-operative UO and AKI postoperatively. However, we did find a significant association when directly comparing non-AKI and severe forms of AKI (stage 2/3) groups. Our data showed a slightly decreasing trend in UO according to the AKI stage (Fig. 2). We think this is not a chance finding; it is consistent with the diagnostic criteria of AKI in terms of UO.14 Maybe only when intra-operative UO falls below a specific threshold does it reveal its true relationship with postoperative renal dysfunction. However, our study sample size was much smaller than those of other studies that have investigated intra-operative UO and postoperative AKI,8,9,24 and we did not obtain a clinical oliguria threshold according to our sample size. Additionally, in our study, the intra-operative UO in patients with mild AKI (2.7 [1.3 to 5.2] ml kg−1 h−1) was similar to those without AKI (2.7 [1.4 to 4.9] ml kg−1 h−1). Children who developed mild AKI account for a large portion of patients with postoperative AKI, and the proportion is approximately 77% (209/273). Thus, the significant association between intra-operative UO and postoperative renal dysfunction may be masked by mild AKI. Furthermore, although previous studies in adults who had undergone cardiac surgery showed that a mild increase in SCr was associated with adverse outcomes,25 mild AKI was not found to be related to short- or long-term adverse outcomes, such as the duration of hospitalisation or long-term renal dysfunction.18,26 In our study, we used the SCr criteria of KDIGO to diagnose AKI. However, children have lower normal baseline SCr values than adults, so the definition of AKI based on the percentage change in creatinine may lead to the misclassification of children without biologically significant kidney injury as having mild AKI, thus influencing the relationship between potential risk factors and renal injury.27 This may partly explain why when we directly compared non-AKI and severe forms of AKI for analysis, a significant association between reduced intra-operative UO and postoperative moderate-to-severe AKI was found. Further large-sample size studies are warranted to explore the statistical differences in intra-operative UO between non-AKI and any stage AKI groups.
Various underlying physiological processes lead to the clinical signs of reduced UO and oliguria, including different fluid administration strategies, haemodynamic changes, and surgical insults during cardiac surgery.28 Previous studies have reported a close relationship between restricted fluid administration and intra-operative reduced UO and adverse outcomes. Those who received restrictive fluid therapy were more likely to have a lower UO than those who received liberal infusion regimens.29,30 Preoperative fasting and other fluid deficits resulting from vasodilation and haemorrhage may also influence intra-operative UO. Additionally, reduced clearance and slower distribution of intra-operative fluids during general anaesthesia result in intra-operative oliguria.14 Intravascular hypovolaemia, prolonged hypotension, reduced perfusion of the kidneys, and non-renal causes such as the release of antidiuretic hormone in response to pain, may also contribute to reduced intra-operative UO.12 Non-pulsatile flow during the process of CPB may induce renal vasoconstriction and redistribution of blood flow away from the kidneys.31 In our study, there were significant differences in the variables measuring peri-operative fluid status between the AKI and non-AKI groups. We controlled for intra-operative fluid administration and blood loss as potential confounders to minimise the influence of input and output fluid on intra-operative UO. Patients who developed postoperative moderate-to-severe AKI had less UO than patients without AKI (2.4 [1.5 to 5.5] and 2.7 [1.4 to 4.9] ml kg−1 h−1, respectively) even if they received more fluid intra-operatively (8.2 [5.6 to 10.9] and 4.7 [3.3 to 7.4] ml kg−1, respectively).
In our study, furosemide was routinely given (923/1184, 78.0%) during the process of CPB. Furosemide is a potent diuretic acting on the sodium-potassium-chloride co-transporter at the intraluminal side of the ascending limb of the loop of Henle, and it is used to promote UO.32 When properly used in fluid overload, furosemide may resolve intra-renal congestion, reduce renal oxygen consumption, and protect against renal injury.33 At our centre, a small dose of furosemide (<5 mg) was given to children during the process of CPB as an approach to avoid oligo-anuria or fluid retention during procedures for congenital heart disease.34 Since it would directly affect the intra-operative UO measurement and potential postoperative kidney function, we adjusted for preoperative and intra-operative diuretic use as confounding factors to minimise their influence. Additionally, a large proportion of patients (990/1184, 83.6%) received modified ultrafiltration after weaning from CPB. The process of ultrafiltration can effectively remove excess water in the body, concentrate blood cells, restore body fluid balance, clear some inflammatory mediators, improve the function of organs after surgery, and improve the clinical effect of cardiac surgery with cardiopulmonary bypass.35,36 Ultrafiltration can affect intra-operative UO by directly influencing the intra-operative volume status and haemodynamic status.37 We also adjusted modified ultrafiltration use as a confounder.
Our data from paediatric cardiac surgery patients that reflects real clinical conditions show that reduced intra-operative UO was associated with postoperative moderate-to-severe AKI after controlling for the above clinically sensitive risk factors. Our results further increase awareness of reduced intra-operative UO as an important sign of postoperative renal injury in daily practice. However, the number of severe AKI cases are limited and may be insufficient to provide a robust conclusion in posthoc analyses. It is crucial to demonstrate a significant association between intra-operative UO and postoperative renal dysfunction in patients with cardiac defects and disturbed haemodynamic status. Studies with larger sample sizes are warranted to determine the oliguria threshold that predicts postoperative AKI in paediatric cardiac surgery.
Our study had several limitations in addition to its retrospective design. First, to guarantee a large sample size, we did not limit the study cohort to special cardiac defects or surgical procedures, and we included the targeted age spectrum to <6 years of age. Approximately half of our children underwent simple cardiac surgery with a shorter surgical time and more modest kidney insults than the others (RACHS-1 score of <3; 685/1184, 57.8%). We also excluded neonates owing to their unique renal physiology, epidemiology and outcomes.38 This may partly explain why our incidence of AKI is a little lower than that reported previously.26 Second, the measurement of intra-operative UO was recorded by medical personnel and extracted from the clinical record, which may be imprecise. Third, we only used the SCr criteria of the KDIGO to diagnose AKI; therefore, there may have been some patients who could have been identified by the UO criteria and were missed. Finally, we were unable to include all potential confounders in this analysis, including radiocontrast administration for cardio-angiography. However, to our best knowledge, very few children have undergone cardiac catheterisation and have been exposed to radiocontrast. Future studies with larger sample sizes that account for more possible confounders or those with prospective designs are warranted.
In conclusion, we did not find a significant association between intra-operative UO and postoperative AKI (stages 1 to 3) in children undergoing surgery for congenital cardiac defects. In the posthoc analyses, we found that reduced intra-operative UO may be correlated with severe postoperative forms of AKI, and reductions in intra-operative urine output below a specific threshold may be associated with post-operative renal dysfunction. Our results may provide clues for future studies and insights into better peri-operative management of paediatric cardiac patients. Large prospective multicentre paediatric studies are warranted to determine the relationship between intra-operative UO and postoperative renal dysfunction (Supplemental Tables).
Supplementary Material
Acknowledgements relating to this article
Assistance with the study: none.
Financial support and sponsorship: this study was supported by the CAMS Innovation Fund for Medical Sciences (2021-I2M-C and T-B-036).
Conflicts of interest: none.
Presentation: none.
This manuscript was handled by Tom Hansen.
Fuxia Yan and Jianhui Wang are co-corresponding authors.
Supplemental digital content is available for this article.
References
- 1.Van den Eynde J, Rotbi H, Schuermans A, et al. Long-term consequences of acute kidney injury after pediatric cardiac surgery: a systematic review. J Pediatr 2023; 252:83–92. e5. [DOI] [PubMed] [Google Scholar]
- 2.Kourelis G, Kanakis M, Samanidis G, et al. Acute kidney injury predictors and outcomes after cardiac surgery in children with congenital heart disease: an observational cohort study. Diagnostics (Basel) 2022; 12:2397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luo XQ, Kang YX, Duan SB, et al. Machine learning-based prediction of acute kidney injury following pediatric cardiac surgery: model development and validation study. J Med Internet Res 2023; 25:e41142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Madsen NL, Goldstein SL, Frøslev T, et al. Cardiac surgery in patients with congenital heart disease is associated with acute kidney injury and the risk of chronic kidney disease. Kidney Int 2017; 92:751–756. [DOI] [PubMed] [Google Scholar]
- 5.Thomas ME, Blaine C, Dawnay A, et al. The definition of acute kidney injury and its use in practice. Kidney Int 2015; 87:62–73. [DOI] [PubMed] [Google Scholar]
- 6.Peng K, McIlroy DR, Bollen BA, et al. Society of Cardiovascular Anesthesiologists clinical practice update for management of acute kidney injury associated with cardiac surgery. Anesth Analg 2022; 135:744–756. [DOI] [PubMed] [Google Scholar]
- 7.Hori D, Katz NM, Fine DM, et al. Defining oliguria during cardiopulmonary bypass and its relationship with cardiac surgery-associated acute kidney injury. Br J Anaesth 2016; 117:733–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhao BC, Lei SH, Yang X, et al. Assessment of prognostic value of intra-operative oliguria for postoperative acute kidney injury: a retrospective cohort study. Br J Anaesth 2021; 126:799–807. [DOI] [PubMed] [Google Scholar]
- 9.Shiba A, Uchino S, Fujii T, et al. Association between intra-operative oliguria and acute kidney injury after major noncardiac surgery. Anesth Analg 2018; 127:1229–1235. [DOI] [PubMed] [Google Scholar]
- 10.Xu X, Nie S, Zhang A, et al. Acute kidney injury among hospitalized children in China. Clin J Am Soc Nephrol 2018; 13:1791–1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neunhoeffer F, Wiest M, Sandner K, et al. Noninvasive measurement of renal perfusion and oxygen metabolism to predict postoperative acute kidney injury in neonates and infants after cardiopulmonary bypass surgery. Br J Anaesth 2016; 117:623–634. [DOI] [PubMed] [Google Scholar]
- 12.Kunst G, Ostermann M. Intra-operative permissive oliguria – how much is too much? Br J Anaesth 2017; 119:1075–1077. [DOI] [PubMed] [Google Scholar]
- 13.Schacham NY, Chhabada S, Efune PN, et al. Intra-operative hypotension and acute kidney injury after noncardiac surgery in infants and children: a retrospective cohort analysis. Anesthesiology 2022; 136:93–103. [DOI] [PubMed] [Google Scholar]
- 14.Van den Eynde J, Delpire B, Jacquemyn X, et al. Risk factors for acute kidney injury after pediatric cardiac surgery: a meta-analysis. Pediatr Nephrol 2022; 37:509–519. [DOI] [PubMed] [Google Scholar]
- 15.Kellum JA, Lameire N. KDIGO AKI Guideline Work Group. Diagnosis, evaluation, and management of acute kidney injury: a KDIGO summary (Part 1). Crit Care 2013; 17:204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim JH. Multicollinearity and misleading statistical results. Korean J Anesthesiol 2019; 72:558–569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chertow GM, Levy EM, Hammermeister KE, et al. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998; 104:343–348. [DOI] [PubMed] [Google Scholar]
- 18.Taylor ML, Carmona F, Thiagarajan RR, et al. Mild postoperative acute kidney injury and outcomes after surgery for congenital heart disease. J Thorac Cardiovasc Surg 2013; 146:146–152. [DOI] [PubMed] [Google Scholar]
- 19.Raina R, Chakraborty R, Tibrewal A, et al. Advances in pediatric acute kidney injury. Pediatr Res 2022; 91:44–55. [DOI] [PubMed] [Google Scholar]
- 20.Salmon AHJ, Satchell SC. Endothelial glycocalyx dysfunction in disease: albuminuria and increased microvascular permeability. J Pathol 2012; 226:562–574. [DOI] [PubMed] [Google Scholar]
- 21.Prowle JR, Kirwan CJ, Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat Rev Nephrol 2014; 10:37–47. [DOI] [PubMed] [Google Scholar]
- 22.Salmasi V, Maheshwari K, Yang D, et al. Relationship between intra-operative hypotension, defined by either reduction from baseline or absolute thresholds, and acute kidney and myocardial injury after noncardiac surgery: a retrospective cohort analysis. Anesthesiology 2017; 126:47–65. [DOI] [PubMed] [Google Scholar]
- 23.Pang Z, Liang S, Xing M, et al. The correlation of intra-operative oliguria with acute kidney injury after noncardiac surgery: a systematic review and meta-analysis. Int J Surg 2023; 109:449–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myles PS, McIlroy DR, Bellomo R, Wallace S. Importance of intra-operative oliguria during major abdominal surgery: findings of the Restrictive versus Liberal Fluid Therapy in Major Abdominal Surgery trial. Br J Anaesth 2019; 122:726–733. [DOI] [PubMed] [Google Scholar]
- 25.Lassnigg A, Schmidlin D, Mouhieddine M, et al. Minimal changes of serum creatinine predict prognosis in patients after cardiothoracic surgery: a prospective cohort study. J Am Soc Nephrol 2004; 15:1597–1605. [DOI] [PubMed] [Google Scholar]
- 26.Alten JA, Cooper DS, Blinder JJ, et al. Epidemiology of acute kidney injury after neonatal cardiac surgery: a report from the multicenter neonatal and pediatric heart and renal outcomes network. Crit Care Med 2021; 49:e941–e951. [DOI] [PubMed] [Google Scholar]
- 27.Waikar SS, Bonventre JV. Creatinine kinetics and the definition of acute kidney injury. J Am Soc Nephrol 2009; 20:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.du Toit L, Biccard BM. The relationship between intra-operative oliguria and acute kidney injury. Br J Anaesth 2019; 122:707–710. [DOI] [PubMed] [Google Scholar]
- 29.Myles PS, Bellomo R, Corcoran T, et al. Restrictive versus liberal fluid therapy for major abdominal surgery. N Engl J Med 2018; 378:2263–2274. [DOI] [PubMed] [Google Scholar]
- 30.Prowle JR, Echeverri JE, Ligabo EV, et al. Fluid balance and acute kidney injury. Nat Rev Nephrol 2010; 6:107–115. [DOI] [PubMed] [Google Scholar]
- 31.Lannemyr L, Bragadottir G, Krumbholz V, et al. Effects of cardiopulmonary bypass on renal perfusion, filtration, and oxygenation in patients undergoing cardiac surgery. Anesthesiology 2017; 126:205–213. [DOI] [PubMed] [Google Scholar]
- 32.Ho KM, Power BM. Benefits and risks of furosemide in acute kidney injury. Anaesthesia 2010; 65:283–293. [DOI] [PubMed] [Google Scholar]
- 33.Joannidis M, Druml W, Forni LG, et al. Prevention of acute kidney injury and protection of renal function in the intensive care unit: update 2017: expert opinion of the Working Group on Prevention, AKI section, European Society of Intensive Care Medicine. Intensive Care Med 2017; 43:730–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Onder AM, Rosen D, Mullett C, et al. Comparison of intra-operative aminophylline versus furosemide in treatment of oliguria during pediatric cardiac surgery. Pediatr Crit Care Med 2016; 17:753–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Talwar S, Sujith NS, Rajashekar P, et al. Modified ultrafiltration and postoperative course in patients undergoing repair of tetralogy of Fallot. J Card Surg 2021; 36:3679–3687. [DOI] [PubMed] [Google Scholar]
- 36.Lang SM, Syed MA, Dziura J, et al. The effect of modified ultrafiltration on angiopoietins in pediatric cardiothoracic operations. Ann Thorac Surg 2014; 98:1699–1704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Türköz A, Tunçay E, Balci ŞT. The effect of modified ultrafiltration duration on pulmonary functions and hemodynamics in newborns and infants following arterial switch operation. Pediatr Crit Care Med 2014; 15:600–607. [DOI] [PubMed] [Google Scholar]
- 38.Selewski DT, Charlton JR, Jetton JG, et al. Neonatal acute kidney injury. Pediatrics 2015; 136:e463–e473. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.