Abstract
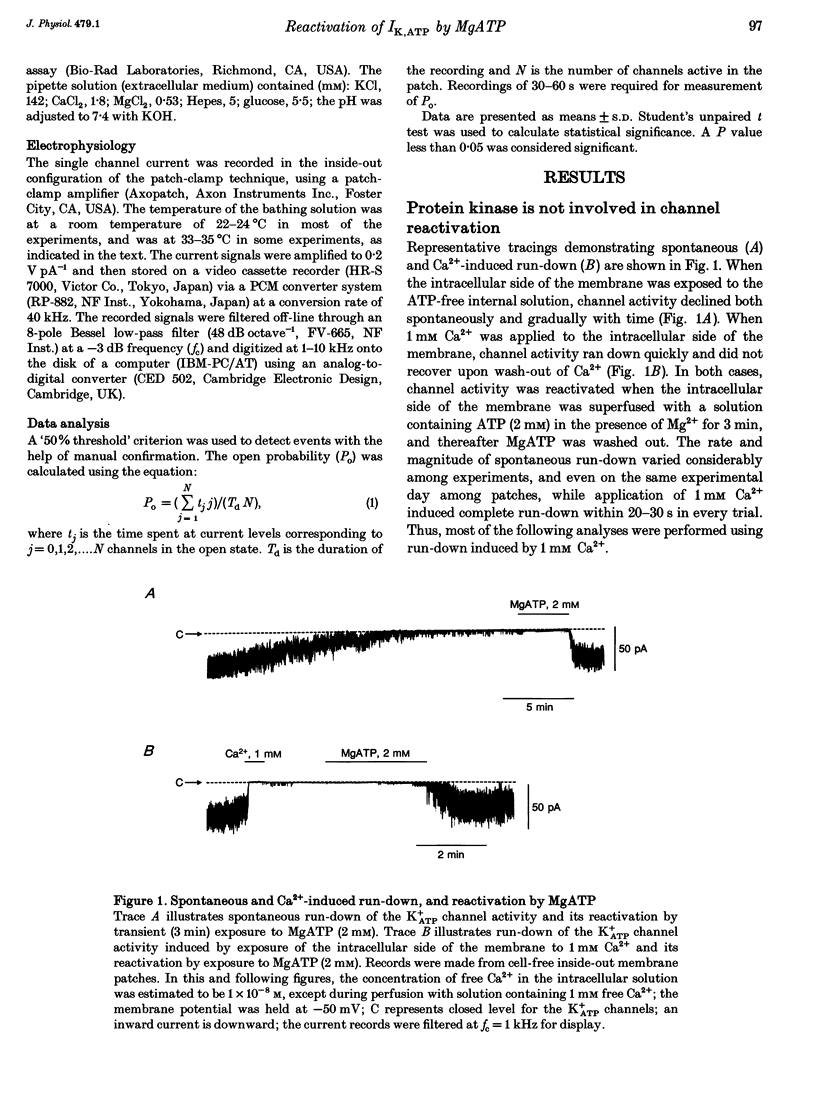
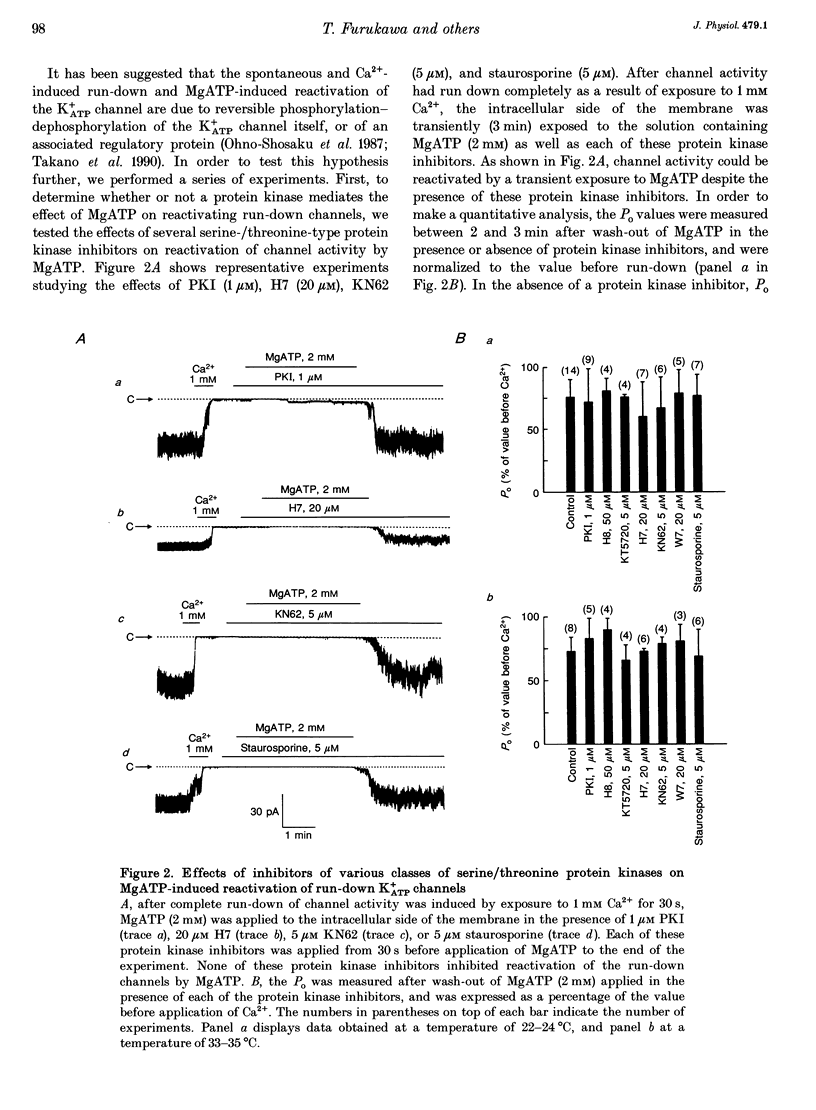
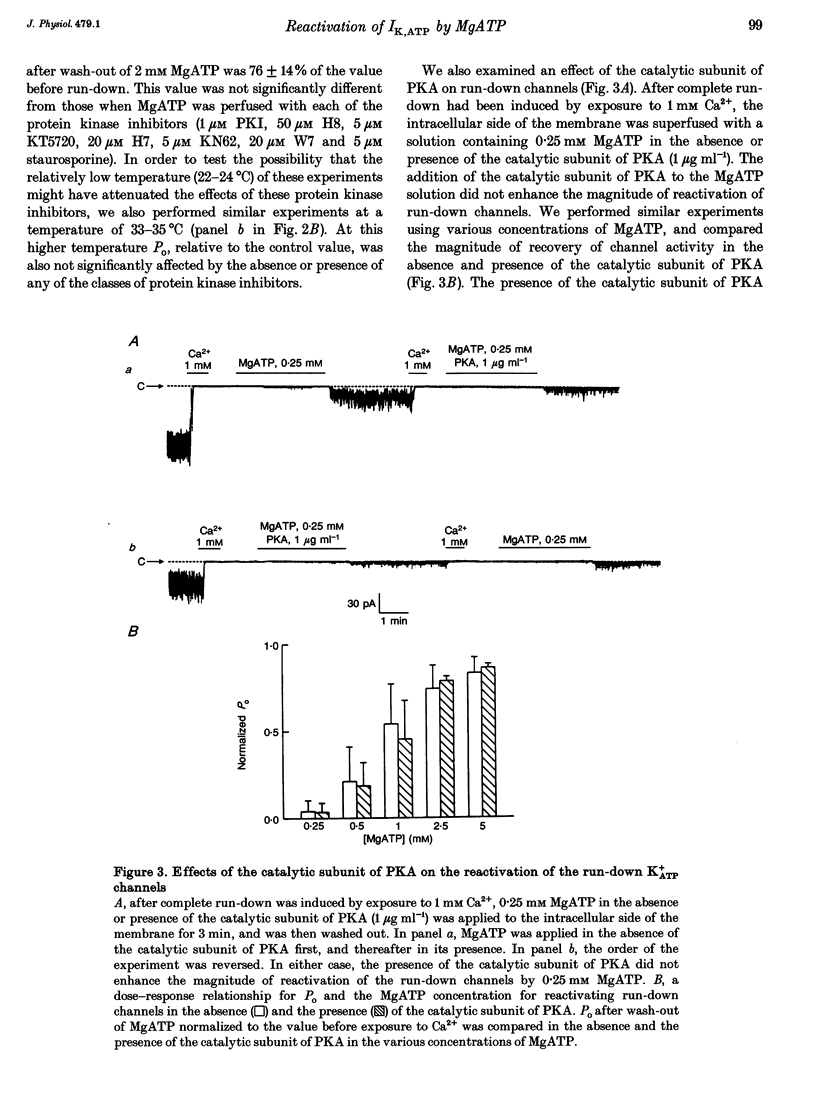
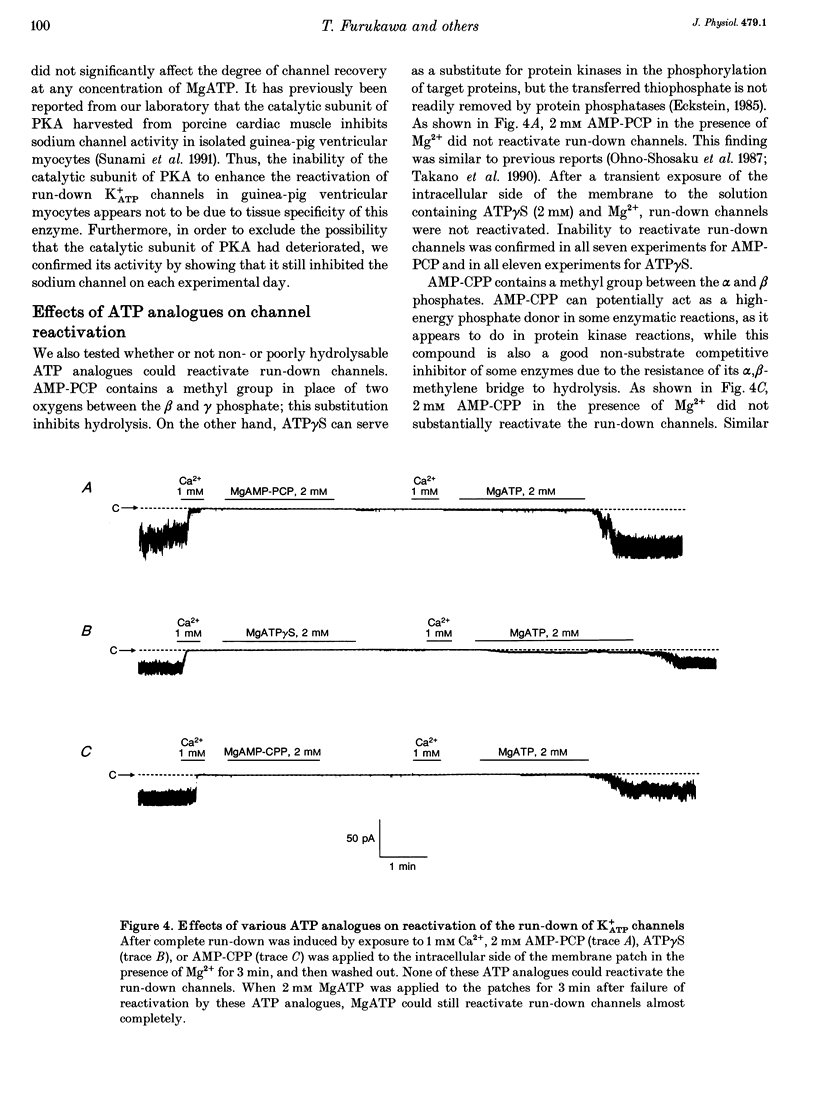
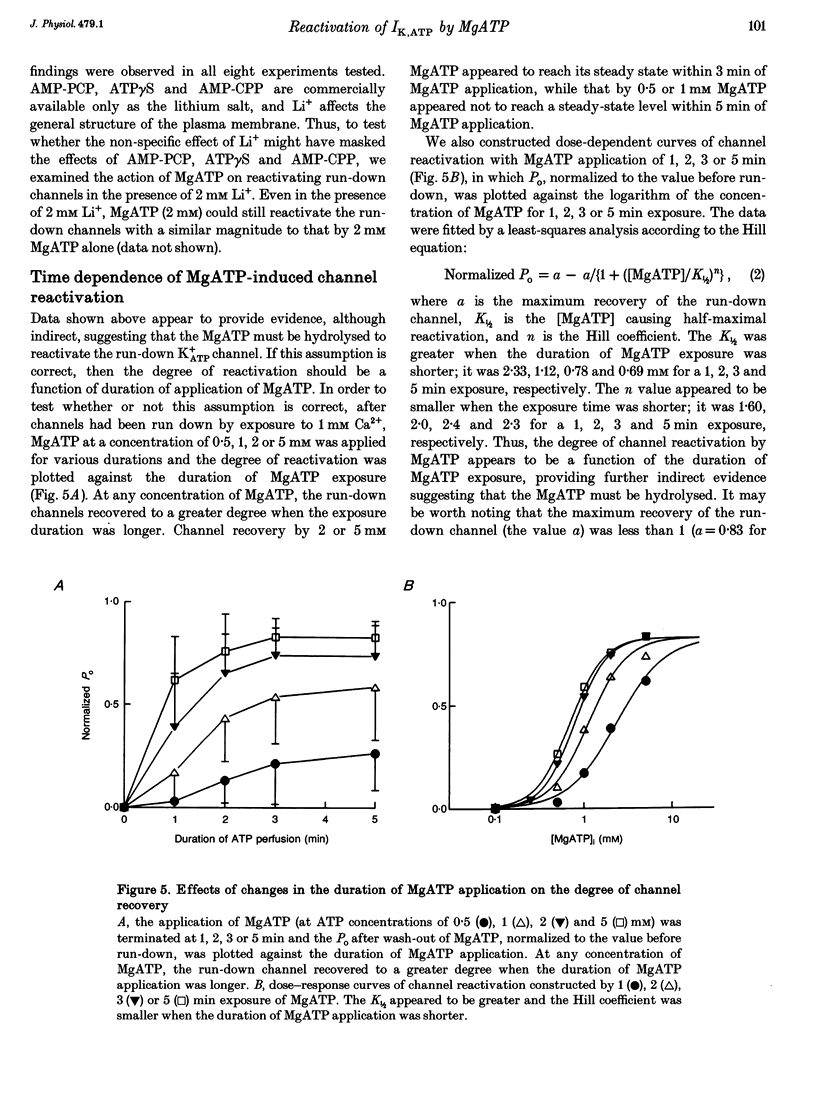
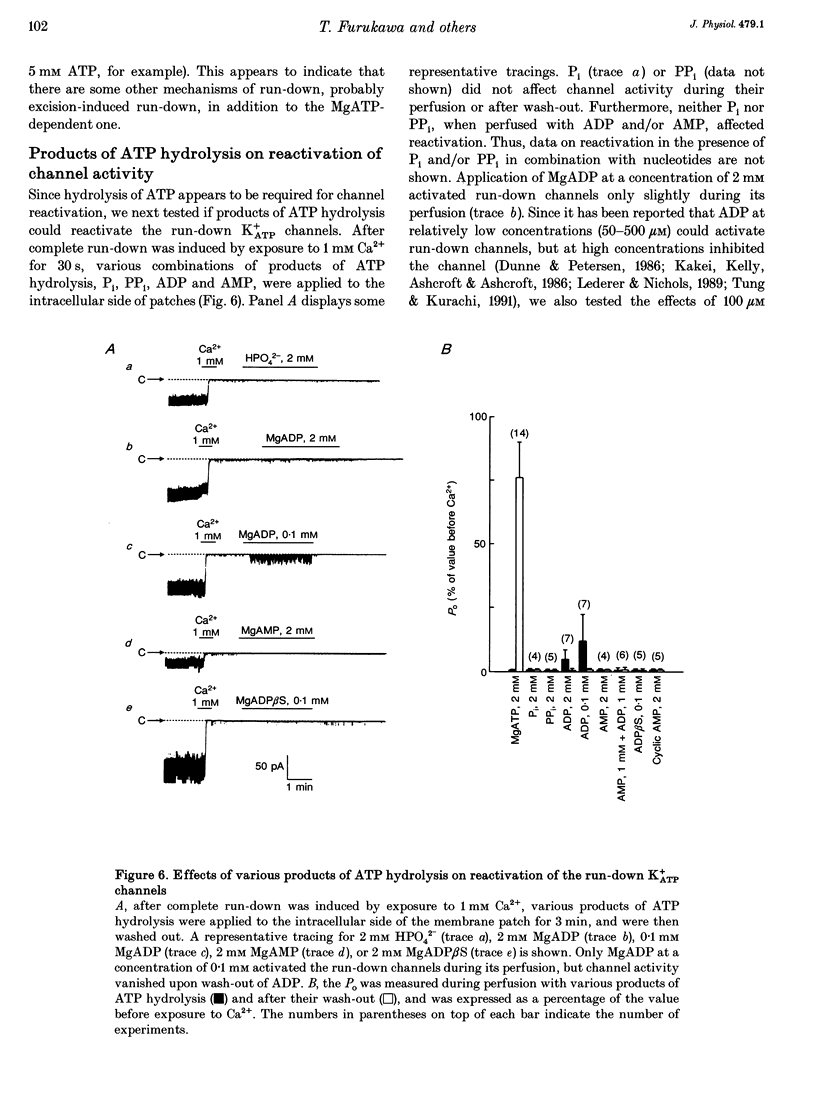
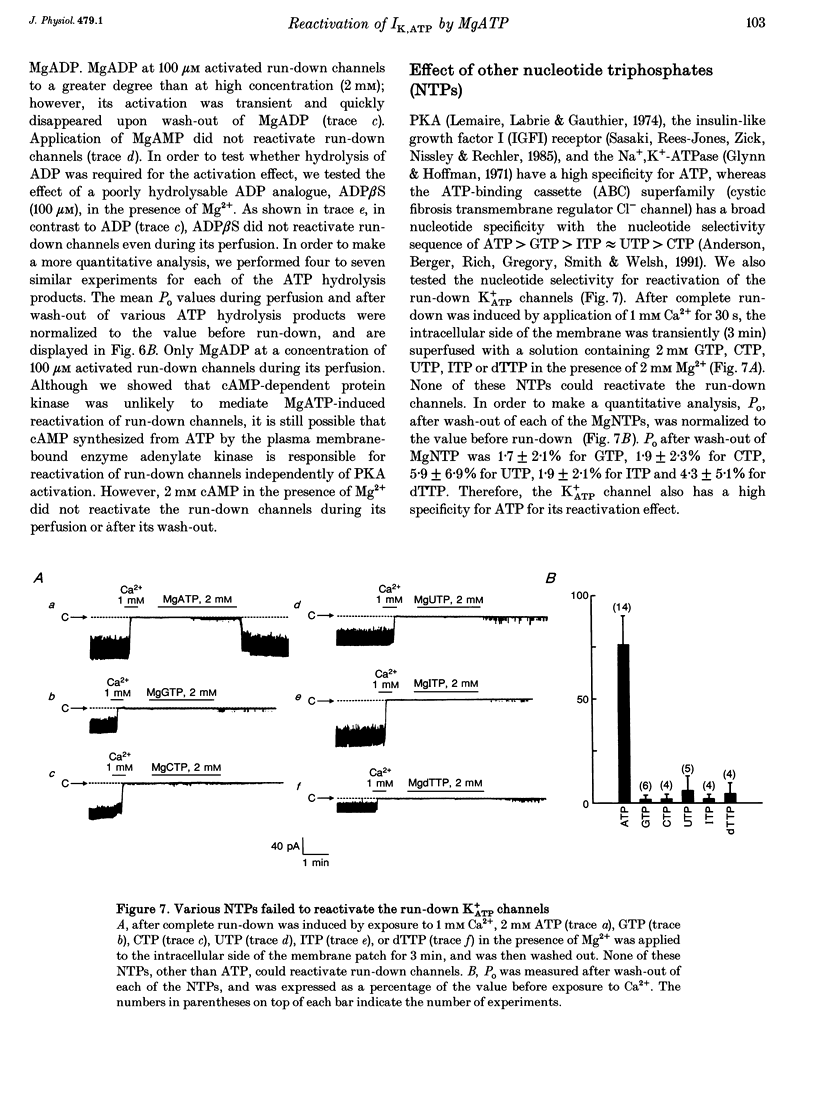
1. A mechanism underlying reactivation of the adenosine 5'-triphosphate-sensitive K+ (K+ATP) channels by MgATP complexes after run-down was examined in guinea-pig ventricular myocytes using the patch-clamp technique with inside-out patch configuration. 2. After run-down was induced by exposure of the intracellular side of the membrane patch to Ca2+ (1 mM), channel activity was reactivated by exposure and subsequent wash-out of MgATP (2 mM). Addition of inhibitors of various serine/threonine protein kinases to the MgATP solution did not suppress reactivation of the run-down channels. 3. Non- or poorly hydrolysable ATP analogues were unable to reactivate run-down channels. 4. The degree of channel recovery was dependent upon the duration of MgATP exposure. The apparent half-activation value (K1/2) of MgATP for reactivation was decreased with increasing exposure time. 5. Various products of ATP hydrolysis were unable to reactivate run-down channels except a relatively low concentration (100 microM) of ADP exposure. 6. Other nucleotide triphosphates, in the presence of Mg2+, were unable to reactivate rundown channels. 7. Fluorescein 5-isothiocyanate (50 microM), which interacts with lysine residues of the nucleotide-binding site on various ATPases, inhibited K+ATP channel activity. After wash-out, channel activity recovered only slightly. 8. These data suggest that the hydrolysis of ATP is important for reactivation of run-down K+ATP channels but that protein phosphorylation by serine/threonine protein kinases may not be involved. Since no products of ATP hydrolysis could reproduce MgATP-induced channel reactivation and since the degree of channel recovery was dependent upon the duration of MgATP application, the hydrolysis energy appears to be utilized for channel reactivation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abbott A. J., Amler E., Ball W. J., Jr Immunochemical and spectroscopic characterization of two fluorescein 5'-isothiocyanate labeling sites on Na+,K(+)-ATPase. Biochemistry. 1991 Feb 12;30(6):1692–1701. doi: 10.1021/bi00220a035. [DOI] [PubMed] [Google Scholar]
- Albitz R., Kammermeier H., Nilius B. Free energy of ATP-hydrolysis fails to affect ATP-dependent potassium channels in isolated mouse ventricular cells. J Mol Cell Cardiol. 1990 Feb;22(2):183–190. doi: 10.1016/0022-2828(90)91114-m. [DOI] [PubMed] [Google Scholar]
- Anderson M. P., Berger H. A., Rich D. P., Gregory R. J., Smith A. E., Welsh M. J. Nucleoside triphosphates are required to open the CFTR chloride channel. Cell. 1991 Nov 15;67(4):775–784. doi: 10.1016/0092-8674(91)90072-7. [DOI] [PubMed] [Google Scholar]
- Beavo J. A., Bechtel P. J., Krebs E. G. Preparation of homogeneous cyclic AMP-dependent protein kinase(s) and its subunits from rabbit skeletal muscle. Methods Enzymol. 1974;38:299–308. doi: 10.1016/0076-6879(74)38046-9. [DOI] [PubMed] [Google Scholar]
- Dunne M. J., Petersen O. H. Intracellular ADP activates K+ channels that are inhibited by ATP in an insulin-secreting cell line. FEBS Lett. 1986 Nov 10;208(1):59–62. doi: 10.1016/0014-5793(86)81532-0. [DOI] [PubMed] [Google Scholar]
- Eckstein F. Nucleoside phosphorothioates. Annu Rev Biochem. 1985;54:367–402. doi: 10.1146/annurev.bi.54.070185.002055. [DOI] [PubMed] [Google Scholar]
- Farley R. A., Faller L. D. The amino acid sequence of an active site peptide from the H,K-ATPase of gastric mucosa. J Biol Chem. 1985 Apr 10;260(7):3899–3901. [PubMed] [Google Scholar]
- Findlay I. Calcium-dependent inactivation of the ATP-sensitive K+ channel of rat ventricular myocytes. Biochim Biophys Acta. 1988 Aug 18;943(2):297–304. doi: 10.1016/0005-2736(88)90561-5. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Petersen O. H. ATP-sensitive inward rectifier and voltage- and calcium-activated K+ channels in cultured pancreatic islet cells. J Membr Biol. 1985;88(2):165–172. doi: 10.1007/BF01868430. [DOI] [PubMed] [Google Scholar]
- Findlay I., Dunne M. J., Ullrich S., Wollheim C. B., Petersen O. H. Quinine inhibits Ca2+-independent K+ channels whereas tetraethylammonium inhibits Ca2+-activated K+ channels in insulin-secreting cells. FEBS Lett. 1985 Jun 3;185(1):4–8. doi: 10.1016/0014-5793(85)80729-8. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Fan Z., Sawanobori T., Hiraoka M. Modification of the adenosine 5'-triphosphate-sensitive K+ channel by trypsin in guinea-pig ventricular myocytes. J Physiol. 1993 Jul;466:707–726. [PMC free article] [PubMed] [Google Scholar]
- Glynn I. M., Hoffman J. F. Nucleotide requirements for sodium-sodium exchange catalysed by the sodium pump in human red cells. J Physiol. 1971 Oct;218(1):239–256. doi: 10.1113/jphysiol.1971.sp009612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho K., Nichols C. G., Lederer W. J., Lytton J., Vassilev P. M., Kanazirska M. V., Hebert S. C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993 Mar 4;362(6415):31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Kakei M., Kelly R. P., Ashcroft S. J., Ashcroft F. M. The ATP-sensitivity of K+ channels in rat pancreatic B-cells is modulated by ADP. FEBS Lett. 1986 Nov 10;208(1):63–66. doi: 10.1016/0014-5793(86)81533-2. [DOI] [PubMed] [Google Scholar]
- Kakei M., Noma A., Shibasaki T. Properties of adenosine-triphosphate-regulated potassium channels in guinea-pig ventricular cells. J Physiol. 1985 Jun;363:441–462. doi: 10.1113/jphysiol.1985.sp015721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlowski R. Z., Ashford M. L. ATP-sensitive K(+)-channel run-down is Mg2+ dependent. Proc R Soc Lond B Biol Sci. 1990 Jun 22;240(1298):397–410. doi: 10.1098/rspb.1990.0044. [DOI] [PubMed] [Google Scholar]
- Lederer W. J., Nichols C. G. Nucleotide modulation of the activity of rat heart ATP-sensitive K+ channels in isolated membrane patches. J Physiol. 1989 Dec;419:193–211. doi: 10.1113/jphysiol.1989.sp017869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaire S., Labrie F., Gauthier M. Adenosine-3',5'-monophosphate-dependent protein kinase from bovine anterior pituitary gland. 3. Structural specificity of the ATP site of the catalytic subunit. Can J Biochem. 1974 Feb;52(2):137–141. doi: 10.1139/o74-021. [DOI] [PubMed] [Google Scholar]
- Muallem S., Karlish S. J. Catalytic and regulatory ATP-binding sites of the red cell Ca2+ pump studied by irreversible modification with fluorescein isothiocyanate. J Biol Chem. 1983 Jan 10;258(1):169–175. [PubMed] [Google Scholar]
- Ohno-Shosaku T., Zünkler B. J., Trube G. Dual effects of ATP on K+ currents of mouse pancreatic beta-cells. Pflugers Arch. 1987 Feb;408(2):133–138. doi: 10.1007/BF00581342. [DOI] [PubMed] [Google Scholar]
- Sasaki N., Rees-Jones R. W., Zick Y., Nissley S. P., Rechler M. M. Characterization of insulin-like growth factor I-stimulated tyrosine kinase activity associated with the beta-subunit of type I insulin-like growth factor receptors of rat liver cells. J Biol Chem. 1985 Aug 15;260(17):9793–9804. [PubMed] [Google Scholar]
- Scott T. L. Distances between the functional sites of the (Ca2+ + Mg2+)-ATPase of sarcoplasmic reticulum. J Biol Chem. 1985 Nov 25;260(27):14421–14423. [PubMed] [Google Scholar]
- Spruce A. E., Standen N. B., Stanfield P. R. Studies of the unitary properties of adenosine-5'-triphosphate-regulated potassium channels of frog skeletal muscle. J Physiol. 1987 Jan;382:213–236. doi: 10.1113/jphysiol.1987.sp016364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturgess N. C., Kozlowski R. Z., Carrington C. A., Hales C. N., Ashford M. L. Effects of sulphonylureas and diazoxide on insulin secretion and nucleotide-sensitive channels in an insulin-secreting cell line. Br J Pharmacol. 1988 Sep;95(1):83–94. doi: 10.1111/j.1476-5381.1988.tb16551.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A., Fan Z., Nakamura F., Naka M., Tanaka T., Sawanobori T., Hiraoka M. The catalytic subunit of cyclic AMP-dependent protein kinase directly inhibits sodium channel activities in guinea-pig ventricular myocytes. Pflugers Arch. 1991 Oct;419(3-4):415–417. doi: 10.1007/BF00371125. [DOI] [PubMed] [Google Scholar]
- Takano M., Qin D. Y., Noma A. ATP-dependent decay and recovery of K+ channels in guinea pig cardiac myocytes. Am J Physiol. 1990 Jan;258(1 Pt 2):H45–H50. doi: 10.1152/ajpheart.1990.258.1.H45. [DOI] [PubMed] [Google Scholar]
- Trube G., Hescheler J. Inward-rectifying channels in isolated patches of the heart cell membrane: ATP-dependence and comparison with cell-attached patches. Pflugers Arch. 1984 Jun;401(2):178–184. doi: 10.1007/BF00583879. [DOI] [PubMed] [Google Scholar]
- Tung R. T., Kurachi Y. On the mechanism of nucleotide diphosphate activation of the ATP-sensitive K+ channel in ventricular cell of guinea-pig. J Physiol. 1991 Jun;437:239–256. doi: 10.1113/jphysiol.1991.sp018593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Weille J. R., Müller M., Lazdunski M. Activation and inhibition of ATP-sensitive K+ channels by fluorescein derivatives. J Biol Chem. 1992 Mar 5;267(7):4557–4563. [PubMed] [Google Scholar]


