Abstract
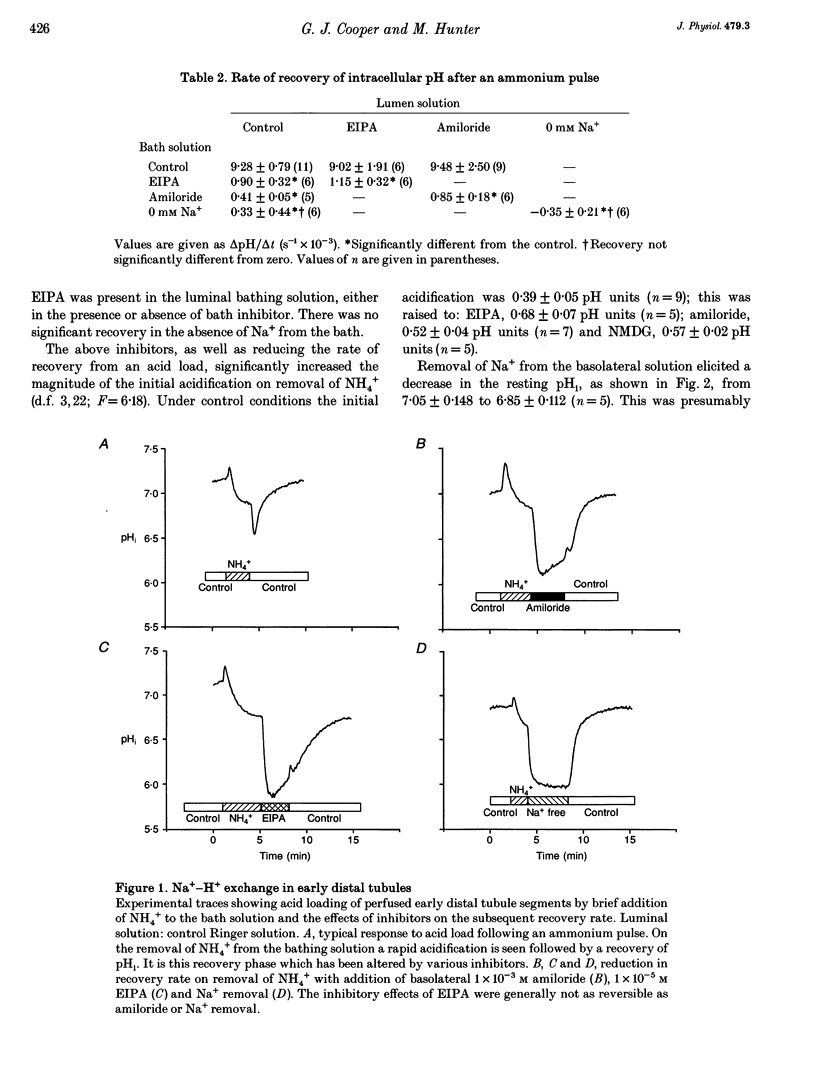
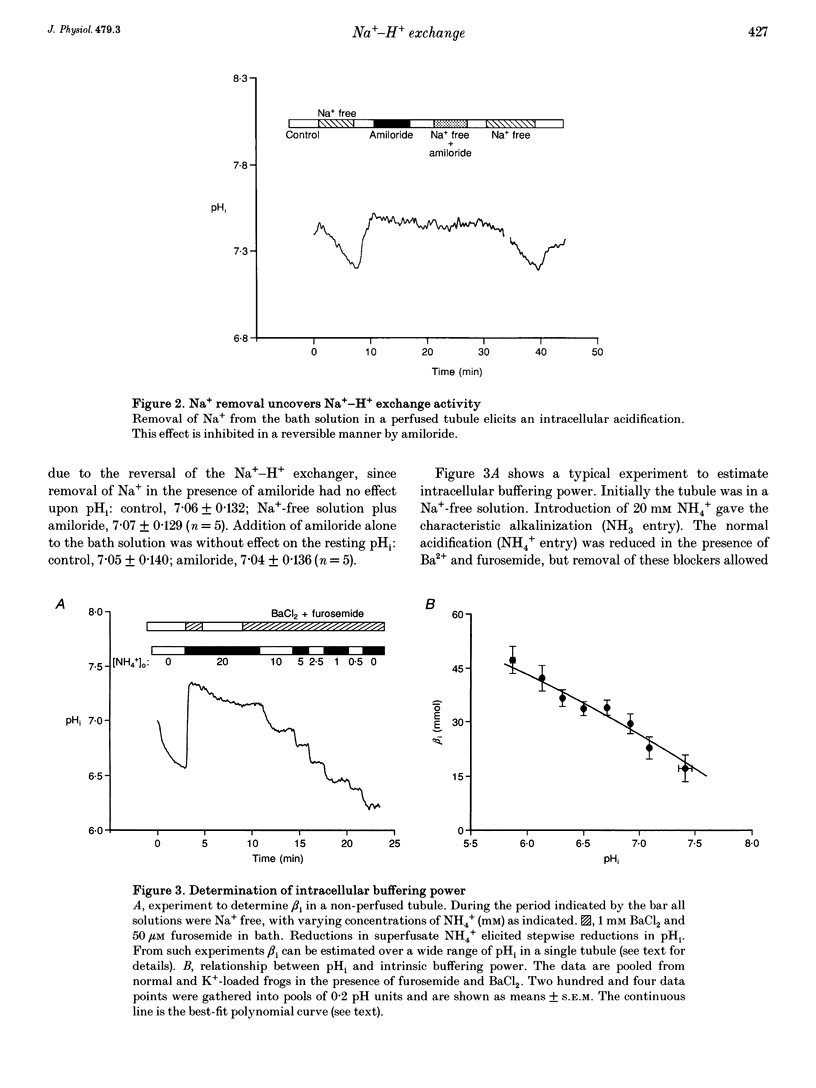
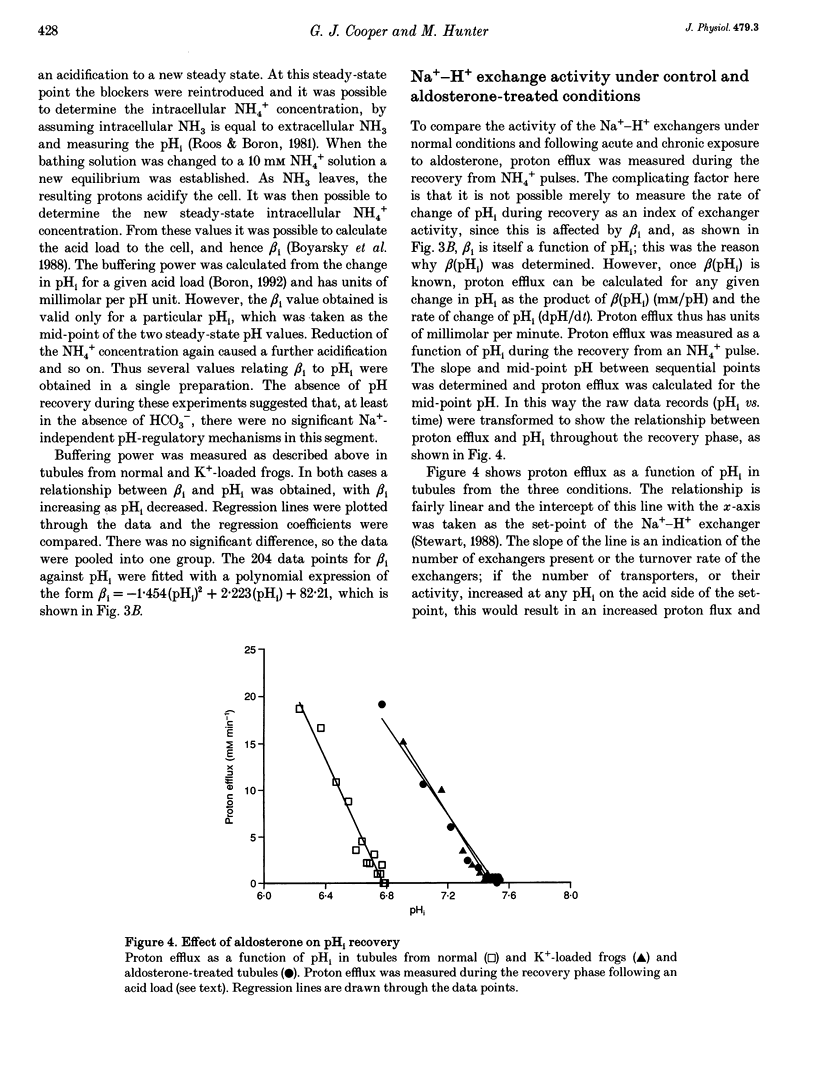
1. Intracellular pH (pHi) regulation was investigated in frog early distal tubule. Single tubules were dissected and perfused, such that the compositions of apical and basolateral solutions could be varied independently. pHi was measured using the fluorescent probe 2',7'-bis(carboxyethyl)-5,6-carboxyfluorescein (BCECF). 2. Brief exposure to NH4+ on the basolateral aspect of the tubules elicited an intracellular acidification, followed by an active recovery. The recovery was inhibited by amiloride and its analogue 5-(N-ethyl-N-isopropyl) amiloride (EIPA) when added to the basolateral, but not the apical, solution. Omission of Na+ from the basolateral solution alone completely inhibited pHi recovery. Thus the Na(+)-H+ exchangers appear to be located on the basolateral membrane. 3. Neither amiloride nor EIPA had any effect on pHi under control conditions, suggesting that the activity of the Na(+)-H+ exchangers at the resting pHi is low. However, removal of basolateral Na+ caused an acidification that was blocked by amiloride, indicating that the Na(+)-H+ exchangers can be activated from the resting state. 4. Intrinsic buffering power (beta i) was determined by stepwise removal of ammonium from the cells in Na(+)-free conditions, to prevent pH regulation, and in the presence of Ba2+ and furosemide (frusemide), to inhibit ammonium transport. beta i was a function of pHi, increasing as pHi decreased. 5. Proton efflux was calculated during the recovery from an acid load in tubules from normal and K(+)-loaded frogs and in tubules which had been incubated for 30 min with aldosterone. Potassium loading produces a chronic increase in plasma aldosterone. Both acute and chronic aldosterone treatment caused an intracellular alkalinization. This was due to an alkaline shift in the set-point of the basolateral Na(+)-H+ exchanger, with no change in the density and/or turnover rate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aronson P. S., Nee J., Suhm M. A. Modifier role of internal H+ in activating the Na+-H+ exchanger in renal microvillus membrane vesicles. Nature. 1982 Sep 9;299(5879):161–163. doi: 10.1038/299161a0. [DOI] [PubMed] [Google Scholar]
- Bonanno J. A., Machen T. E. Intracellular pH regulation in basal corneal epithelial cells measured in corneal explants: characterization of Na/H exchange. Exp Eye Res. 1989 Jul;49(1):129–142. doi: 10.1016/0014-4835(89)90081-x. [DOI] [PubMed] [Google Scholar]
- Boron W. F., Boulpaep E. L. Intracellular pH regulation in the renal proximal tubule of the salamander. Na-H exchange. J Gen Physiol. 1983 Jan;81(1):29–52. doi: 10.1085/jgp.81.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F., De Weer P. Intracellular pH transients in squid giant axons caused by CO2, NH3, and metabolic inhibitors. J Gen Physiol. 1976 Jan;67(1):91–112. doi: 10.1085/jgp.67.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boron W. F. Intracellular pH transients in giant barnacle muscle fibers. Am J Physiol. 1977 Sep;233(3):C61–C73. doi: 10.1152/ajpcell.1977.233.3.C61. [DOI] [PubMed] [Google Scholar]
- Boyarsky G., Ganz M. B., Sterzel R. B., Boron W. F. pH regulation in single glomerular mesangial cells. I. Acid extrusion in absence and presence of HCO3-. Am J Physiol. 1988 Dec;255(6 Pt 1):C844–C856. doi: 10.1152/ajpcell.1988.255.6.C844. [DOI] [PubMed] [Google Scholar]
- Burg M., Grantham J., Abramow M., Orloff J. Preparation and study of fragments of single rabbit nephrons. Am J Physiol. 1966 Jun;210(6):1293–1298. doi: 10.1152/ajplegacy.1966.210.6.1293. [DOI] [PubMed] [Google Scholar]
- Chang E. B., Musch M. W., Drabik-Arvans D., Rao M. C. Phorbol ester inhibition of chicken intestinal brush-border sodium-proton exchange. Am J Physiol. 1991 Jun;260(6 Pt 1):C1264–C1272. doi: 10.1152/ajpcell.1991.260.6.C1264. [DOI] [PubMed] [Google Scholar]
- Garritsen A., Ijzerman A. P., Tulp M. T., Cragoe E. J., Jr, Soudijn W. Receptor binding profiles of amiloride analogues provide no evidence for a link between receptors and the Na+/H+ exchanger, but indicate a common structure on receptor proteins. J Recept Res. 1991;11(6):891–907. doi: 10.3109/10799899109064686. [DOI] [PubMed] [Google Scholar]
- Graber M. L., DiLillo D. C., Friedman B. L., Pastoriza-Munoz E. Characteristics of fluoroprobes for measuring intracellular pH. Anal Biochem. 1986 Jul;156(1):202–212. doi: 10.1016/0003-2697(86)90174-0. [DOI] [PubMed] [Google Scholar]
- Greger R. Ion transport mechanisms in thick ascending limb of Henle's loop of mammalian nephron. Physiol Rev. 1985 Jul;65(3):760–797. doi: 10.1152/physrev.1985.65.3.760. [DOI] [PubMed] [Google Scholar]
- Grinstein S., Rothstein A. Mechanisms of regulation of the Na+/H+ exchanger. J Membr Biol. 1986;90(1):1–12. doi: 10.1007/BF01869680. [DOI] [PubMed] [Google Scholar]
- Guggino W. B., Oberleithner H., Giebisch G. The amphibian diluting segment. Am J Physiol. 1988 May;254(5 Pt 2):F615–F627. doi: 10.1152/ajprenal.1988.254.5.F615. [DOI] [PubMed] [Google Scholar]
- Hurst A. M., Hunter M. Acute changes in channel density of amphibian diluting segment. Am J Physiol. 1990 Dec;259(6 Pt 1):C1005–C1009. doi: 10.1152/ajpcell.1990.259.6.C1005. [DOI] [PubMed] [Google Scholar]
- Krapf R. Basolateral membrane H/OH/HCO3 transport in the rat cortical thick ascending limb. Evidence for an electrogenic Na/HCO3 cotransporter in parallel with a Na/H antiporter. J Clin Invest. 1988 Jul;82(1):234–241. doi: 10.1172/JCI113576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minuth W. W., Steckelings U., Gross P. Complex physiological and biochemical action of aldosterone in toad urinary bladder and mammalian renal collecting duct cells. Ren Physiol. 1988;10(6):297–310. doi: 10.1159/000173139. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Potassium transport in the early distal tubule of Amphiuma kidney. Effects of potassium adaptation. Pflugers Arch. 1983 Mar 1;396(3):185–191. doi: 10.1007/BF00587854. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Guggino W., Giebisch G. Resistance properties of the diluting segment of Amphiuma kidney: influence of potassium adaptation. J Membr Biol. 1985;88(2):139–147. doi: 10.1007/BF01868428. [DOI] [PubMed] [Google Scholar]
- Oberleithner H. Intracellular pH in diluting segment of frog kidney. Pflugers Arch. 1985 Jul;404(3):244–251. doi: 10.1007/BF00581246. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Kersting U., Hunter M. Cytoplasmic pH determines K+ conductance in fused renal epithelial cells. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8345–8349. doi: 10.1073/pnas.85.21.8345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Messner G., Wang W. Mechanism of hydrogen ion transport in the diluting segment of frog kidney. Pflugers Arch. 1984 Nov;402(3):272–280. doi: 10.1007/BF00585510. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Lang F., Wang W., Messner G., Deetjen P. Evidence for an amiloride sensitive Na+ pathway in the amphibian diluting segment induced by K+ adaptation. Pflugers Arch. 1983 Nov;399(3):166–172. doi: 10.1007/BF00656710. [DOI] [PubMed] [Google Scholar]
- Oberleithner H., Weigt M., Westphale H. J., Wang W. Aldosterone activates Na+/H+ exchange and raises cytoplasmic pH in target cells of the amphibian kidney. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1464–1468. doi: 10.1073/pnas.84.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roos A., Boron W. F. Intracellular pH. Physiol Rev. 1981 Apr;61(2):296–434. doi: 10.1152/physrev.1981.61.2.296. [DOI] [PubMed] [Google Scholar]
- Stanton B., Biemesderfer D., Stetson D., Kashgarian M., Giebisch G. Cellular ultrastructure of Amphiuma distal nephron: effects of exposure to potassium. Am J Physiol. 1984 Sep;247(3 Pt 1):C204–C216. doi: 10.1152/ajpcell.1984.247.3.C204. [DOI] [PubMed] [Google Scholar]
- Stewart D. J. Sodium-proton exchanger in isolated hepatocytes exhibits a set point. Am J Physiol. 1988 Sep;255(3 Pt 1):G346–G351. doi: 10.1152/ajpgi.1988.255.3.G346. [DOI] [PubMed] [Google Scholar]
- Thomas J. A., Buchsbaum R. N., Zimniak A., Racker E. Intracellular pH measurements in Ehrlich ascites tumor cells utilizing spectroscopic probes generated in situ. Biochemistry. 1979 May 29;18(11):2210–2218. doi: 10.1021/bi00578a012. [DOI] [PubMed] [Google Scholar]
- Wang W. H., Henderson R. M., Geibel J., White S., Giebisch G. Mechanism of aldosterone-induced increase of K+ conductance in early distal renal tubule cells of the frog. J Membr Biol. 1989 Nov;111(3):277–289. doi: 10.1007/BF01871012. [DOI] [PubMed] [Google Scholar]
- Wang W., Dietl P., Oberleithner H. Evidence for Na+ dependent rheogenic HCO3- transport in fused cells of frog distal tubules. Pflugers Arch. 1987 Mar;408(3):291–299. doi: 10.1007/BF02181472. [DOI] [PubMed] [Google Scholar]
- Wehling M., Eisen C., Christ M. Aldosterone-specific membrane receptors and rapid non-genomic actions of mineralocorticoids. Mol Cell Endocrinol. 1992 Dec;90(1):C5–C9. doi: 10.1016/0303-7207(92)90092-k. [DOI] [PubMed] [Google Scholar]
- Wehling M., Käsmayr J., Theisen K. Fast effects of aldosterone on electrolytes in human lymphocytes are mediated by the sodium-proton-exchanger of the cell membrane. Biochem Biophys Res Commun. 1989 Nov 15;164(3):961–967. doi: 10.1016/0006-291x(89)91763-4. [DOI] [PubMed] [Google Scholar]


