Dear Editor-in-Chief
Carbapenem has been recognized as one of the most important last-resort drugs against infections caused by Gram-negative pathogenic bacteria (1). However, the efficacy and potency of carbapenem are increasingly threatened by the continued emergence and proliferation of carbapenem-inactivating enzymes, some of serine β-lactamases (SBLs) and all of metallo-β-lactamases (MBLs) (2). MBLs as carbapenemases, are perceived as a more pressing clinical concern than SBLs due to several reasons. First, there is currently no clinically approved inhibitor for MBLs. Second, clinically relevant subclass B1 MBLs such as New Delhi MBL (NDM), imipenemase (IMP), and Verona integron-encoded MBL (VIM), are frequently horizontally transferred. Third, novel polymorphic variants of MBLs which exhibit an extended spectrum of resistance, continue to emerge (2). Since the identification of the first type, NDM-1, 55 variants have been deposited in the β-lactamase database (BLDB) as of May 2023 (3). To combat bacterial infections caused by multidrug-resistant Gram-negative pathogens carrying these deadly antibiotic resistance determinants, several chemotherapeutic regimens have been introduced in clinical settings (4). However, most approaches are facing the emergence of unexpected resistance, emphasizing the urgent need to develop MBL inhibitors as a direct countermeasure to control the resistance (2, 4).
Significant efforts have been made to develop novel broad-spectrum MBL inhibitors (4). Currently, only three MBL inhibitor candidates are undergoing clinical trials and all of them are boronate compounds that mimic the transient tetrahedral intermediate binding during β-lactam hydrolysis (4). Taniborbactam (VNRX-5133) is in phase 3 clinical trials and other boronate compounds, xeruborbactam (QPX7728) and QPX7831 are in phase 1 (4). Taniborbactam demonstrates the inhibitory activity against various MBLs except for IMP (5), and the combination of cefepime and taniborbactam has shown significant effectiveness against carbapenem-resistant Gram-negative isolates (6). However, a very recent study reported the pre-existence of resistance against this promising drug. A variant called NDM-9, which carries a single amino acid substitution (Glu152Lys) compared to NDM-1, exhibited reduced inhibition by taniborbactam, and recombinant Escherichia coli strain harboring blaNDM-9 gene showed dramatic resistance against the cefepime-taniborbactam combination (7).
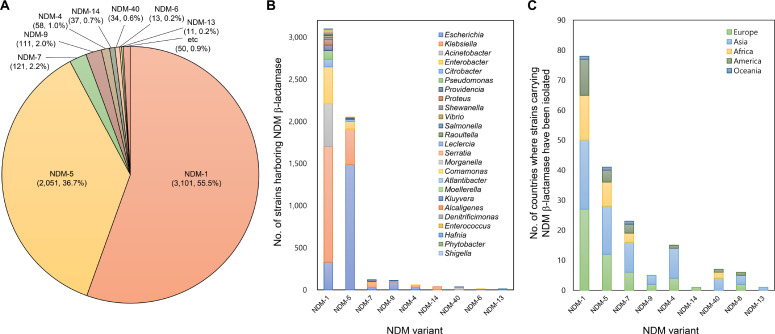
Similar results were observed when the single mutation on the corresponding position (Glu163Lys) was introduced to VIM-2 MBL (7). To understand the menace posed by NDM-9, an investigation was conducted on the prevalence and distribution of NDM variants using the NCBI RefSeq genome database. As of May 2023, 5587 NDM proteins were found in bacterial genomes. The proportion of major NDM variants are as follows: NDM-1 (55.5%), NDM-5 (36.7%), NDM-7 (2.2%), and NDM-9 (2.0%) (Fig. 1A). Analyses of taxonomic distribution revealed that the predominant taxonomic origin of NDM-9 was E. coli (85.6%), with limited occurrences in Klebsiella pneumoniae (11.7%), Salmonella enterica (1.8%), and Acinetobacter baumannii (0.9%). In contrast, the other major variants exhibited a broader taxonomic distribution, with NDM-1, NDM-5, and NDM-7 identified in 23, 11, and 6 genera, respectively (Fig. 1B). Analyses of geographical distribution also showed the similar pattern as NDM-9 was found only in five European and Asian countries, whereas NDM-1, NDM-5, and NDM-7 were found in 78, 41, and 23 countries across all continents, respectively (Fig. 1C).
Fig. 1:
The prevalence and distribution of NDM variants in bacterial genomes of the NCBI RefSeq genome database, A) Number of NDM variants and their proportion in the genome database, B) Taxonomic distribution of strains harboring NDM variants, C) Geographical distribution of strains carrying NDM variants
NDM-9 is relatively less prevalent than other major variants and has not spread widely across various taxa and geographical locations. Notably, no other variants with the substitution at position 152 were found in the database, suggesting that the mutation of NDM-9 has not been descended extensively, although the variant is one of the early emerged variants. On the contrary, some other substitutions in major variants were conserved in recently emerged variants which display higher resistance (8). Indeed, the minimal inhibitory concentration values of NDM-9 for carbapenem antibiotics were reduced compared to other variants (8). The substitution of NDM-9 may not provide a selective advantage within current chemotherapeutic regimens. The introduction of taniborbactam in clinical settings has the potential to selective NDM-9 over other major variants, leading to the dissemination of this presently less prevalent variant. The introduction of taniborbactam could accelerate the dissemination of NDM-9, thereby intensifying clinical concerns associated with the variant. Additionally, the genotyping of NDM variants prior to taniborbactam treatment is crucial to mitigate the risk of clinical failure.
Acknowledgements
This work was supported by research grants from the Bio & Medical Technology Development Program of the National Research Foundation of Korea (NRF) funded by the Ministry of Science, Information and Communications Technology (MSIT; grant No. NRF-2017M3A9E4078014); and from the NRF funded by the MSIT (grant Nos. NRF-2021R1A2C3004826 and NRF-2019R1C1C1008615). The funders had no influence on the design, collection, analysis and interpretation of the data, writing of the report, and decision to submit this article for publication.
Footnotes
Conflict of interest
The authors declare that there is no conflict of interests.
References
- 1.Papp-Wallace KM, Endimiani A, Taracila MA, et al. (2011). Carbapenems: past, present, and future. Antimicrob Agents Chemother, 55(11):4943–4960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mojica MF, Rossi MA, Vila AJ, et al. (2022). The urgent need for metallo-β-lactamase inhibitors: an unattended global threat. Lancet Infect Dis, 22(1):e28–e34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Naas T, Oueslati S, Bonnin RA, et al. (2017). Beta-lactamase database (BLDB) - structure and function. J Enzyme Inhib Med Chem, 32(1):917–919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang Y, Yan YH, Schofield CJ, et al. (2023). Metallo-β-lactamase-mediated antimicrobial resistance and progress in inhibitor discovery. Trends Microbiol, 31(7):735–748. [DOI] [PubMed] [Google Scholar]
- 5.Liu B, Trout REL, Chu GH, et al. (2020). Discovery of taniborbactam (VNRX-5133): a broad-spectrum serine- and metallo-β-lactamase inhibitor for carbapenem-resistant bacterial infections. J Med Chem, 63(6):2789–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Karlowsky JA, Hackel MA, Wise MG, et al. (2023). In vitro activity of cefepimetaniborbactam and comparators against clinical isolates of Gram-negative Bacilli from 2018 to 2020: results from the global evaluation of antimicrobial resistance via surveillance (GEARS) program. Antimicrob Agents Chemother, 67(1):e0128122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Le Terrier C, Gruenig V, Fournier C, et al. (2023). NDM-9 resistance to taniborbactam. Lancet Infect Dis, 23(4):401–402. [DOI] [PubMed] [Google Scholar]
- 8.Wang X, Li H, Zhao C, et al. (2014). Novel NDM-9 metallo-β-lactamase identified from a ST107 Klebsiella pneumoniae strain isolated in China. Int J Antimicrob Agents, 44(1):90–91. [DOI] [PubMed] [Google Scholar]