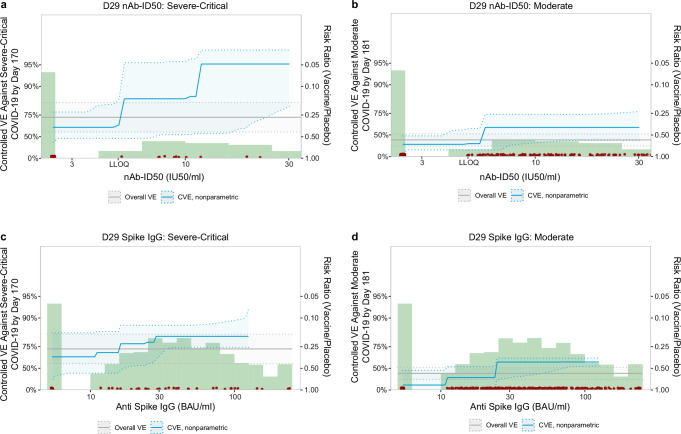
Fig. 4. Vaccine efficacy against severe-critical COVID-19 or against moderate COVID-19 by D29 antibody marker level.
Vaccine efficacy estimates against (a, c) severe-critical COVID-19 and against (b, d) moderate COVID-19 through 170 (severe-critical) or 181 (moderate) days post-D29 were obtained using a nonparametric implementation of the method of Gilbert et al.30. Each point on the curve represents the estimated controlled vaccine efficacy at the given D29 antibody marker level: (a, b) 50% inhibitory dilution neutralizing antibody (nAb-ID50) titer and (c, d) anti-Spike IgG binding antibody concentration. Dotted lines indicate bootstrap pointwise 95% CIs. The green histograms are frequency distributions of D29 marker level, with maroon dots representing marker levels of individual cases. Analyses adjusted for baseline behavioral risk score and geographic region. Curves are plotted over the nAb-ID50 titer range from unquantifiable to the 90th percentile (30.2 IU50/ml) and over the Spike IgG concentration range from negative response to the 90th percentile (125 BAU/ml). The horizontal gray line is the overall vaccine efficacy against (a, c) severe-critical COVID-19 or against (b, d) moderate COVID-19 through 170 (severe-critical) or 181 (moderate) days post-D29, with the dotted gray lines indicating the 95% CIs. BAU binding antibody units, CVE controlled vaccine efficacy, IU international units, LLOQ lower limit of quantitation, nAb-ID50 50% inhibitory dilution neutralizing antibody. nAb-ID50 LLOQ = 4.8975 IU50/ml; Spike IgG positivity cutoff = 10.8424 BAU/ml. Source data are provided as a Source Data file.