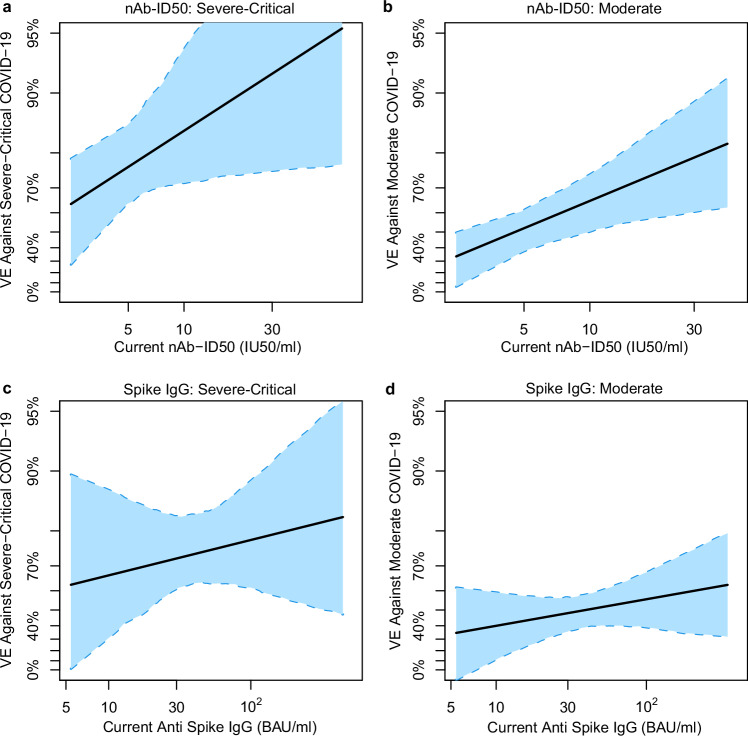
Fig. 5. Exposure-proximal vaccine efficacy against severe-critical COVID-19 or against moderate COVID-19 by current antibody marker level.
Analyses were performed in baseline SARS-CoV-2 seronegative per-protocol vaccine recipients. Exposure-proximal vaccine efficacy estimates against (a, c) severe-critical COVID-19 and against (b, d) moderate COVID-19 through 170 (severe-critical) or 181 (moderate) days post-D29 by current antibody marker level were obtained using the method of Huang and Follmann44, with “current” referring to the true underlying antibody marker level not subject to technical measurement error, in a hypothetical scenario in which the value was available from serum samples collected every day over the follow-up period (see “Methods”). Each point on the curve represents the vaccine efficacy at the given current antibody marker level: (a, b) 50% inhibitory dilution neutralizing antibody (nAb-ID50) titer and (c, d) anti-Spike IgG binding antibody concentration. The dashed lines are bootstrap pointwise 95% CIs. Analyses adjusted for baseline behavioral risk score and geographic region. Curves are plotted over the range from negative binding antibody response (or unquantifiable neutralizing antibody titer) to the 97.5th percentile of each current antibody marker level: Spike IgG, negative response to 352 BAU/ml; RBD IgG, negative response to 486 BAU/ml; nAb-ID50, unquantifiable to 43.4 IU50/ml. Positivity cutoffs: 10.8424 BAU/ml for Spike and 14.0858 BAU/ml for RBD; nAb-ID50 LLOQ = 4.8975 IU50/ml. BAU binding antibody units, CI confidence interval, IU international units, LLOQ lower limit of quantitation, nAb-ID50 50% inhibitory dilution neutralizing antibody. Source data are provided as a Source Data file.