FIGURE 5.
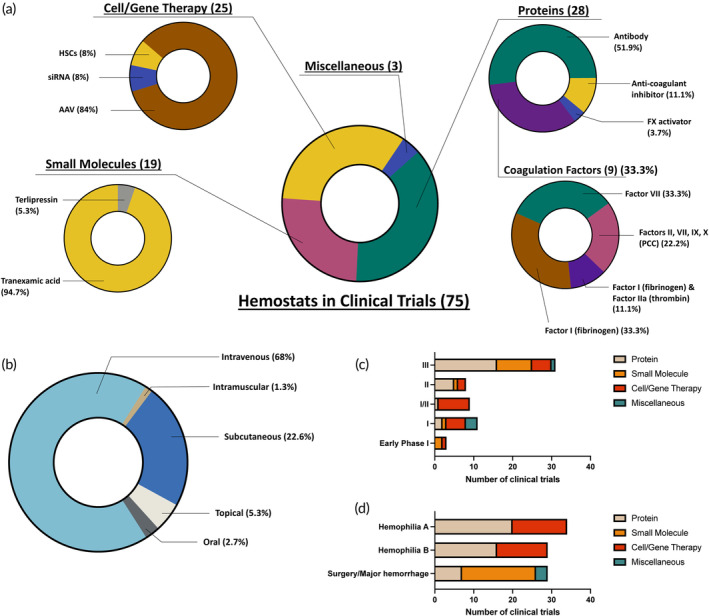
Hemostats in clinical trials. (a) Modality of investigational hemostats. (b) Route of administration of investigational hemostats. (c) Number of clinical trials of hemostats per indication. (d) Number of clinical trials of hemostats per phase. AAV, adeno‐associated virus; HSCs, hematopoietic stem cells; siRNA, small interfering RNA; vWD, von Willebrand disease. vWF, von Willebrand factor.
