Abstract
Hydrogels have been used in the clinic since the late 1980s with broad applications in drug delivery, cosmetics, tissue regeneration, among many other areas. The past three decades have witnessed rapid advances in the fields of polymer chemistry, crosslinking approaches, and hydrogel fabrication methods, which have collectively brought many new hydrogel products, either injectable or non‐injectable, to clinical studies. In an article published in 2020 entitled “Hydrogels in the clinic”, we reviewed the clinical landscape and translational challenges of injectable hydrogels. Here, we provide an update on the advances in the field and also extend the scope to include non‐injectable hydrogels. We highlight recently approved hydrogel products, provide an update on the clinical trials of injectable hydrogels, and discuss active clinical trials of topically applied and implantable hydrogels.
Keywords: clinic, clinical translation, clinical trials, crosslinking, drug delivery, hydrogel, injectable materials, tissue engineering, translational medicine
Translational Impact Statement.
This review provide an update on the clinical landscape of injectable and non‐injectable hydrogels, highlighting medical products based on hydrogels that have been FDA/EMA approved or investigated in active clinical trials.
1. INTRODUCTION
Hydrogels remain one of the most widely studied technologies in the clinic with a wide spectrum of biomedical applications such as in drug delivery and tissue engineering. The clinical landscape of hydrogels continues to rapidly evolve, driven by the invention of new hydrogels, addition of new functionalities to existing hydrogels, approval of new hydrogel products, and the growing need of hydrogels in solving emerging clinical problems.
In a review article published in 2020, 1 we reviewed the clinical landscape of injectable hydrogels, an important subset of hydrogels broadly used or investigated in the clinic. Our analysis revealed that the primary clinical applications of bulk hydrogel biomaterials were soft contact lenses (202 active trials in 2020), with topically applied hydrogel materials (e.g., wound dressings, 99 active trials in 2020), injectable hydrogels for tissue augmentation or regeneration (116 trials in 2020), and hydrogel‐based coils for endovascular embolization (8 trials in 2020) each being evaluated to a less frequent extent. Thirty‐six percent of clinically approved injectable hydrogels and 26% of clinical trials involving hydrogel products at that time incorporated a bioactive agent for drug delivery. Focusing our detailed analysis on a subset of hydrogel products—injectable hydrogels—we highlighted 28 injectable hydrogel‐based products approved by the US Food and Drug Administration (FDA) and/or European Medicines Agency (EMA). We detailed 31 active clinical trials investigating injectable hydrogels for regenerative medicine, cancer care, urinary incontinence, and ocular applications. We also summarized a set of biological, technological, and regulatory challenges that have limited the translation of injectable hydrogels.
As a 4‐year update to our last review, we extend our discussions to include both injectable and non‐injectable hydrogels, covering additional topics such as hydrogel‐based implants, topical treatments/wound dressings, and drug delivery devices. As a result of this expanded definition, we now highlight over 100 approved products and 210 active clinical trials of both injectable and non‐injectable hydrogels. This updated review highlights the widespread clinical studies of hydrogels in some important biomedical applications, in not only the prevailing applications of cosmetic surgery, musculoskeletal regenerative medicine, skin wound healing, optometry, and cancer treatment, but also the emerging areas of surgical adhesion prevention, implant‐associated infection treatment, transdermal drug delivery, female reproductive health, and male birth control. Collectively, this article provides an updated snapshot of the current clinical landscape of hydrogels in 2024.
2. CLARIFYING DEFINITIONS AND IMPORTANT CONSIDERATIONS FOR THERAPEUTIC HYDROGEL PRODUCTS
Here, we defined a “therapeutic hydrogel product” as a material comprised of crosslinked (physical, chemical, or otherwise) polymers, which is swollen in aqueous solution, and is designed to address a medical problem. Our working definition allowed us to properly refine our analysis, excluding viscous gel products, non‐aqueous gels, and products that are not indicated to treat diseases. Our analysis revealed that the backbone polymers for clinical hydrogels continue to be sourced from natural or synthetic sources, including a limited number of composites that combine natural and synthetic polymers. Only five polymers, hyaluronic acid (HA), silicone, poly(ethylene glycol) (PEG), collagen, and cellulose, account for more than half of the approved hydrogel products and more than half of the hydrogels in current clinical trials. The remaining products and trials use other biomaterials.
There are unique design considerations for hydrogel products, both approved and emerging. 2 , 3 , 4 Hydrogel products are formed through crosslinking, covalent or physical, of hydrophilic polymers in aqueous solution. Hydrogels are suitable carriers for the encapsulation and delivery of diverse drugs or biologics, ranging from small molecule anesthetics, to hormones, whole proteins, and stem cells. Hydrogel products, distinctively from other classes of therapeutic biomaterials, are typically designed to integrate with nearby tissue, mimic tissue mechanical properties, support niches with biomimetic pH and ionic strength. 5 , 6 Therapeutic hydrogels participate in a dynamic interplay (i.e., infiltration, modification, and/or degradation) with the tissue surrounding their injection, implantation, or topical application.
The design considerations for hydrogel products pose a particular challenge for cumulative analyses on hydrogel products. Hydrogel device parameters, such as the polymer volume fraction of individual hydrogel backbone components, extent to which a backbone polymer is chemically modified, and the extent of crosslinking achieved are typically proprietary and rarely disclosed on approved product information sheets or clinical trial records. Other descriptive information on the hydrogels' individual polymeric components (e.g., polymer molecular weight and polydispersity), precursor rheology (e.g., viscosity), and crosslinked hydrogels (e.g., storage and loss modulus under shear, elastic modulus under compression, or tangent of the phase angle) are also not provided. The hydrogel dose or dose range for individual clinical trials is also often omitted, even when the precise dose of loaded drugs or therapeutics are provided. Limited availability of these important hydrogel design and delivery parameters limits otherwise fruitful opportunities for meta‐analysis, linking hydrogel design to clinical outcome. If these data were made available in the future, it could enable researchers to abstract new and important structure–function–outcome relationships for therapeutic hydrogels and human patients.
In our following summaries of approved and investigatory hydrogel products, we highlight key available aspects of each device (e.g., biomaterial, natural or synthetic material origin, route of administration, therapeutics delivered).
3. APPROVED PRODUCTS
Over 100 hydrogel products, derived from natural materials, synthetic materials, or their combination, have been approved by the FDA and/or EMA for tissue regeneration, tissue augmentation, facial correction, contact lens and other ocular applications, wound dressing, and as drug delivery devices. An updated list of approved hydrogels is shown in Table 1. Table 1 is a representative, but not exhaustive list, as there are many similar approved products in each category. We highlighted recently approved products.
TABLE 1.
Approved hydrogel products grouped by broad indications and materials.
| Name (company) | Hydrogel material/payload | Administration method | Approved indication | Approval year | |
|---|---|---|---|---|---|
| Injectable hydrogel products | |||||
| Facial correction: synthetic | Radiesse®(+) (Merz Pharmaceutical) a | Hydroxylapatite, carboxymethylcellulose with lidocaine | Dermis | Correction of wrinkles and folds, stimulation of natural collagen production | FDA (2015) |
| Radiesse® (Bioform Medical, Inc.) a | Hydroxylapatite, carboxymethylcellulose | Dermis | Correction of facial folds and wrinkles, signs of facial fat loss and volume loss | EMA (2004), FDA (2006 for first indication) | |
| Artefill® (Suneva Medical, Inc.) a | Polymethylmethacrylate beads, collagen, and lidocaine | Dermis | Facial wrinkles and folds | FDA (2006) | |
| Bellafill® PMMA Collagen Dermal Filler (Suneva Medical, Inc.) | Polymethylmethacrylate beads, Bovine collagen, and lidocaine | Dermis | Wrinkles and acne scars | FDA (2006 for first indication) | |
| Ellansé (Sinclair) | Polycaprolactone (PCL) microspheres in carboxymethyl cellulose hydrogel | Dermis | Correction of wrinkles and folds, stimulation of natural collagen production | CE (2009) | |
| Facial correction: natural | Belotero balance®(+) Lidocaine (Merz Pharmaceuticals) a | Hyaluronic acid with lidocaine | Dermis | Moderate to severe facial wrinkles and folds | FDA (2019) |
| Revanesse® Versa, Revanesse® Ultra (Prollenium Medical Technologies, Inc.) a | Hyaluronic acid | Dermis | Moderate to severe facial wrinkles and folds | FDA (2017) | |
| Revanesse® Versa+ (Prollenium Medical Technologies, Inc.) a | Hyaluronic acid with lidocaine | Dermis | Moderate to severe facial wrinkles and folds | FDA (2018) | |
| Revanesse® Lips+ (Prollenium Medical Technologies, Inc.) | Hyaluronic acid | Lips | Restore lost volume and create a fuller‐looking lip | FDA (2020) | |
| Teosyal® RHA (Teoxane SA) a | Hyaluronic acid | Dermis | Facial wrinkles and folds | EMA (2015), FDA (2017) | |
|
Restylane® Lyft, Restylane® Refyne, Restylane® Defyne (Galderma Laboratories, L.P.) a Restylane® Silk (Valeant Pharmaceuticals North AmericaLLC/Medicis) a Restylane® Injectable Gel (Medicis Aesthetics Holdings, Inc.) a Restylane® Kysse, Restylane® Eyelight (Galderma Laboratories, L.P.) |
Hyaluronic acid with lidocaine | Subcutaneous, dermis, lips | For correction of volume deficit, facial folds and wrinkles, midface contour deficiencies, perioral rhytids, and infraorbital hollowing | EMA (2010), FDA (2012 for first indication, 2023 for last indication) | |
| Belotero balance® (Merz Pharmaceuticals) a | Hyaluronic acid | Dermis | Moderate to severe facial wrinkles and folds | EMA (2004), FDA (2011 for first indication) | |
| Juvéderm® XC (Allergan, Inc.) a | Hyaluronic acid with lidocaine | Facial tissue | Correction of facial wrinkles and folds | FDA (2010) | |
| Evolence® Collagen Filler (Colbar Lifescience) a | Collagen | Dermis | Moderate to deep facial wrinkles and folds | EMA (2004), FDA (2008) | |
| Elevess® (Anika Therapeutics) a | Hyaluronic acid with lidocaine | Dermis | Moderate to severe facial wrinkles and folds | FDA (2006), EMA (2007) | |
| Juvéderm® Voluma XC/Ultra XC/Volbella XC/Vollure XC/VOLBELLA XC/VOLUX XC (Allergan, Inc.) a | Hyaluronic acid | Facial tissue, cheek, lips | For correction of facial wrinkles and folds, volume loss, lip augmentation, undereye hollows, and jawline definition | EMA (2000), FDA (2006 for first indication, 2022 for last indication) | |
| Hylaform® (Hylan B gel), Captique Injectable Gel, Prevelle Silk (Genzyme Biosurgery) a | Modified hyaluronic acid derived from a bird (avian) source | Dermis | Correction of moderate to severe facial wrinkles and folds | EMA (1995), FDA (2004) | |
| Collagen Implant, CosmoDerm® 1 human‐based collagen, CosmoDerm® 2 human‐based collagen CosmoPlast® human‐based collagen (Inamed Corporation/Allergan, Inc.) a | Human collagen | Superficial papillary dermis | For correction of soft tissue contour deficiencies, such as wrinkles and acne scars | FDA and EMA (2003) | |
| Fibrel® (Serono Laboratories) a | Collagen | Dermis | For correction of depressed cutaneous scars | FDA (1988) | |
| Zyplast(R)® and Zyderm(R)® (Inamed Corporation/Allergan, Inc.) a | Bovine collagen | Dermis | For correction of contour deficiencies | FDA and EMA (1981) | |
| RHA® 2, 3, 4, Redensity (Teoxane S.A.) | Hyaluronic acid, 1,4‐butanediol diglycidyl ether with lidocaine | Dermis | Correction of moderate to severe dynamic perioral rhytids | FDA (2017 for first indication, 2021 for last indication) | |
| SKINVIVE by JUVÉDERM (Allergan) | Hyaluronic acid, 1,4‐butanediol diglycidyl ether with lidocaine | Intradermal | Improve the smoothness of the cheeks | FDA (2023) | |
| Tissue fusion, repair and regeneration: synthetic | Emdogain (Straumann) | Porcine enamel matrix derivative in propylene glycol alginate gel | Flap incision or flapless injection | Regenerates periodontal tissue (cementum, periodontal ligament, bone) | FDA (1996) |
| PerioGlas (NovaBone) | Calcium phosphosilicate particles, a PEG and glycerine gel‐like binder | Injection | Dental bone regeneration | FDA (2005) | |
| Actifuse (Baxter) | Phase‐pure silicon‐substituted calcium phosphate in poloxamer 407 | Injection | Bone void filler in spinal and orthopedic application | FDA (2018) | |
| Dynagraft II (IsoTis Orthobiologics) | Demineralized bone matrix in poloxamer | Injection | Bone void filler | FDA (2005) | |
| AlloFuse Plus Paste, AlloFuse Plus Putty (AlloSource) | Allographic demineralized bone matrix in polyethylene oxide polypropylene oxide block copolymer | Spinal fusion or injection to trauma area | Void filler, graft extender | FDA (2011) | |
| Optium DBM Gel (LifeNet Health) | Allographic demineralized bone matrix in glycerol | Spinal fusion or injection to trauma area | Bone graft extender and void filler | FDA (2005) | |
| Grafton DBM gel (Medtronic) | Allographic demineralized bone matrix in glycerol | Spinal fusion or injection to trauma area | Bone graft extender and void filler | FDA (2005) | |
| Arthrosamid (Contura International) | Polyacrylamide | Intraarticular | Treatment of pain in osteoarthritis (OA) | CE(2007), FDA (2014) | |
| GelrinC® (Regentis Biomat.) | PEG diacrylate with denatured fibrinogen | Intraarticular injection after microfracture | Treatment of focal cartilage lesions | CE (2017) | |
| Tissue fusion, repair and regeneration: natural | INFUSE® bone graft (Medtronic Sofamor Danek USA, Inc.) a | Collagen and recombinant human bone morphogenetic protein‐2 | Spinal injection | Spinal fusion, and spine, oral‐maxillofacial and orthopedic trauma surgeries | FDA (2002 for first indication) |
| Osteogenic protein 1 (OP‐1®) implant, OP‐1® Putty (Stryker Biotech) a | Collagen, carboxymethylcellulose, and recombinant OP‐1 | Spinal injection | Posterolateral lumbar spinal fusion | FDA (2001) | |
| Algisyl‐LVR® Hydrogel Implant (LoneStar Heart, Inc.) a | Alginate | Percutaneous | Advanced heart failure | EMA (2014) | |
| Tactoset (Anika Therapeutics) | Hyaluronic acid with calcium phosphate | Injection | Bone void filler for orthopedic application, augmentation of hardware and support of bone fragments during surgery | FDA (2019 for first indication) | |
| Kinex Bioactive Gel (Globus Medical) | Bioglass, collagen and hyaluronic acid | Injection | Bone void filler | FDA (2013) | |
| EUFLEXXA® (Ferring Pharmaceuticals, Inc.) a | Hyaluronic acid | Intra‐articular | Treatment of pain in osteoarthritis (OA) | FDA (2004), EMA (2005) | |
| Gel‐One (Zimmer Biomet) | Cinnamic acid functionalized hyaluronic acid | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | FDA (2011) | |
| Monovisc (Anika Therapeutics) | High MW hyaluronic acid lightly crosslinked with biscarbodiimide | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | CE (2007), FDA (2014) | |
| Cingal (Anika Therapeutics) | High MW hyaluronic acid lightly crosslinked with biscarbodiimide and triamcinolone hexacetonide | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | CE (2016) | |
| Hymovis (Fidia Farmaceutici) | Hyaluronic acid 500–730 kDa, functionalized with 2%–3% hexadecylamine | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | FDA (2015) | |
| TRIVISC (Orthogenrx, Inc.) | Hyaluronic acid | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | FDA (2017) | |
| SINOVIAL (IBSA INSTITUT BIOCHIMIQUE SA) | Hyaluronic acid | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | FDA (2014) | |
| SYNOJOYNT (Arthrex, Inc.) | Hyaluronic acid | Intraarticular injection | Treatment of pain in osteoarthritis (OA) | FDA (2018) | |
| Orthovisc (Anika Therapeutics) | Hyaluronic acid | Intraarticular | Treatment of pain in osteoarthritis (OA) | FDA (2004) | |
| BST‐CarGel® (Smith & Nephew) | Chitosan | Mini‐arthrotomy or arthroscopy | Cartilage repair | CE (2012) | |
| Drug delivery device: synthetic | Jelmyto (UroGen Pharma) | Pluronic F‐127, PEG‐400, HPMC/mitomycin | Catheter instillation | Low‐grade upper tract urothelial cancer | FDA (2020) |
| Vantas® (Endo Pharmaceuticals) a | Histrelin acetate, poly(2‐hydroxyethyl methacrylate), poly(2‐hydroxypropylmethacrylate) and gonadotropin releasing hormone | Subcutaneous | Palliative treatment of prostate cancer | FDA (2004), EMA (2005) | |
| Others | TraceIT® Hydrogel Tissue Marker (Augmenix, Inc.) a | Polyethylene glycol | Percutaneous | Improved soft tissue alignment for image‐guided therapy | FDA (2013) |
| Supprelin LA® (Indevus Pharmaceuticals, Inc.) a | Histrelin acetate, poly(2‐hydroxyethyl methacrylate) | Subcutaneous | Central precocious puberty | EMA (2005), FDA (2007) | |
| Bulkamid® hydrogel (Searchlight Pharma) a | Polyacrylamide | Transurethral | Female stress urinary incontinence | EMA (2003), FDA (2006) | |
| Coaptite® (BioForm Medical, Inc.) a | Calcium hydroxylapatite, sodium carboxymethylcellulose, glycerin | Submucosal | Female stress urinary incontinence | EMA (2001), FDA (2005) | |
| SpaceOAR® Hydrogel (Augmenix, Inc.) a | Polyethylene glycol | Percutaneous | For protecting vulnerable tissues during prostate cancer radiotherapy | EMA (2010), FDA (2015) | |
| Solesta® (Oceana Therapeutics, Inc.) | Hyaluronic Acid (NASHA) and dextranomer (Dx) | Injection | Fecal incontinence | FDA (2011) | |
| Non‐injectable hydrogel products | |||||
| Drug delivery device: synthetic | Cervidil (Ferring Laboratories) | PEG 8000, dicyclohexyl methane‐4, 4′‐diisocyanate and 1,2,6‐hexanetriol/dinoprostone | Vaginal insert | Initiation and/or continuation of cervical ripening in pregnant women at or near term | FDA (1993) |
| Zuplenz TM (Galena Bipharma) | PEG 1000, polyvinyl alcohol and rice starch/Ondansetron | Oral gel | Chemotherapy, radiation, and postoperative‐induced nausea and vomiting | FDA (1991) | |
| Tissue repair: natural | Apligraf (Organogenesis) | Collagen with fibroblast and keratinocytes | Petroleum jelly–impregnated gauze | Diabetic foot ulcer and venous leg ulcers | FDA (2000) |
| GRAFTJACKET Now (Wright Medical Group) | Collagen with different cells | Surgical implant | Tendon and ligamentous tissue repair | FDA (2010) | |
| OrthADAPT (Synovis Orthopedic and Woundcare) | Collagen with different cells | Implant | Attaching tissue to bone, tendon repair | FDA (2005) | |
| Permaco (Covidien) | Collagen | Implant | Tendon and ligamentous repair, surgical implant for ventral hernia repair and abdominal wall reconstruction | FDA (2005) | |
| TissueMend (Stryker) | Collagen | Implant | Tendon and ligamentous repair | FDA (2006) | |
| Zimmer Collagen Repair Patch (Tissue Science Laboratories) | Collagen | Implant | Rotator cuff and tendon repair | FDA (2006) | |
| OrthADAPT® Bioimplant (Pegasus Biologics) | Collagen | Surgical mesh | Attachment of tissue to bone, tendon repair | FDA (2012) | |
| TissueMend® (TEI Biosciences) | Collagen | Implant | Tendon and ligament repair | FDA (2002) | |
| Zimmer® Collagen Repair Patch (Zimmer Biomet) | Collagen | Implant | Rotator cuff and tendon repair | FDA (2002) | |
| Permacol® (Medtronic) | Porcine decellularized matrix | Implant | Tendon and ligament repair, surgical implant for ventral hernia repair and abdominal wall reconstruction | FDA (1999) | |
| Tissue repair: synthetic | DuraSeal (Duraseal) | PEG ester, trilysine amine, decahydrated sodium borate, and others | Surgical implant | Prevention of cerebrospinal fluid leakage after cranial and spinal surgery | FDA (2005) |
| Contact lenses and other ocular applications: synthetic | Airsoft™ (Maxvue Vision) | Silicone | Contact lens | Astigmatism | CE (2009) |
| Abiliti Overnigh Therapeutic Lenses, ACUVUE OASYS (Johnson & Johnson) | Silicone | Contact lens | Myopia and/or hyperopia | FDA (2021); FDA (2018) | |
| Clariti 1 day (Cooper Vision) | Silicone | Contact lens | Short sight and long sight | FDA (2018) | |
| MiSight 1 Day (BenQ) | Omafilcon A | Contact lens | Myopia | FDA (2019) | |
| Dailies AquaComfort (Ciba Vision) | Nelfilcon A | Contact lens |
Astigmatism; optical correction of refractive ametropia |
FDA (2018) | |
| Proclear (Omafilcon B) (Cooper Vision) | 2‐Hydroxyethylmethacrylate and 2‐methacryloxyethyl phosphorylcholine, ethylene glycol dimethacrylate | Contact lens | Daily wear for correction of visual acuity | FDA (2013) | |
| Resure Sealant (Ocular Therapeutix) | PEG | Topical | Intraoperative management of clear corneal incisions following cataract surgery | FDA (2014) | |
| Restasis (Allergan) | Carbomer copolymer type A with cyclosporine | Topical/eye drop | Increase tear production | FDA (2002) | |
| Yutiq (EyePoint Pharmaceuticals) | Polyvinyl alcohol with fluocinolone acetonide | Intravitreal implant | Chronic non‐infectious uveitis affecting the posterior segment of the eye | FDA (2018) | |
| Ozurdex (Allergen) | PLGA with dexamethasone | Intravitreal implant | Macular edema, non‐infectious uveitis | FDA (2009) | |
| Iluvien (Alimera Sciences) | Polyvinyl alcohol, silicone adhesives with fluocinolone acetonide | Intravitreal implant | Diabetic macular edema | FDA (2014) | |
| Azasite (Merck) | Poloxamer407, polycarbophil with azithromycin | Topical/eyedrop | Bacterial conjunctivitis | FDA (2007) | |
| BromSite (Sun Pharmaceutical Industries, Inc.) | Poloxamer407, polycarbophil with bromfenac sodium sesquihydrate | Topical/Ocular instillation | Postoperative inflammation and prevention of ocular pain in patients undergoing cataract surgery | FDA (2016) | |
| Besivance (Bausch + Lomb) | Poloxamer407, polycarbophil with besifloxacin | Topical/Ocular instillation | Bacterial conjunctivitis | FDA (2009) | |
| Bausch + Lomb ULTRA (Bausch + Lomb) | Samfilcon A | Contact lens | Near‐sightedness, far‐sightedness, and astigmatism | FDA (2018) | |
| SBL‐3 Multifocal Intraocular Lens (Lenstec, Inc.) | Hydrophilic acrylic | Intraocular | Vision correction after the eye's natural lens is removed because of cataract | FDA (2022) | |
| Precision7; Precision7 for Astigmatism; Precision7 Multifocal; Precision7 Multifocal Toric (Alcon) | Senofilcon A | Contact lens | Myopia, hyperopia, astigmatism | FDA (2023) | |
| Timoptic XE (Bausch + Lomb) | Anionic heteropolysaccharide with timolol maleate | Topical/eyedrop | Elevated intraocular pressure in patients with ocular hypertension or open‐angle glaucoma | FDA (1993) | |
| EVO ICL and EVO TICL (STAAR Surgical Company) | Collamer | Intraocular | Astigmatism and nearsighted | FDA (2022) | |
| Visian® Toric ICL (STAAR Surgical Company) | Collamer | Intraocular | Myopia and astigmatism | FDA (2018) | |
| Wound dressing: synthetic | 3 M™ Tegaderm™ hydrogel wound filler (3 M) | Propylene glycol | Dermal wound filler | Low to moderate draining wounds, partial and full‐thickness dermal ulcers | FDA (2018) |
| Cutimed gel (BSN Medical) | Carbomer 940 | Wound dressing | Management of dry to low exuding wounds | FDA (2014) | |
| Woun'Dres (Coloplast) | Carbomer, collagen | Wound dressing | Dry wounds | FDA (1999) | |
| DermaSyn (DermaRite Industries) | Carbomer940 | Wound dressing | Management of acute or chronic partial and full thickness wounds/ulcers that are dry or have minimal exudate | FDA (2006) | |
| Suprasorb G (Lohmann & Rauscher Global) | CMC polymer, propylene glycol | Bandage | Dry wounds, lower leg ulcer, pressure ulcer, first and second‐degree burns, scalds | FDA (2016) | |
| DermaGauze™ (DermaRite industries) | Acrylate polymer | Hydrogel impregnated gauze | Acute or chronic partial and full thickness wounds | FDA (2014) | |
| Simpurity™ Hydrogel (Safe n'Simple) | Polyethylene oxide, polyvinyl alcohol, acrylate, polyurethane | Absorben sheet | Dry wounds, skin burns and dry scabs | FDA (2011) | |
| AquaDerm™ (DermaRite industries) | 2‐Acrylamido‐2 methyl‐1‐propanesulfonic acid sodium, propylene glycol, poly (ethylene glycol) dimethacrylate, 2‐hydroxy‐2‐methylpropiophenone | Hydrogel sheet | Pressure ulcers, minor burns and radiation tissue damage | FDA (2013) | |
| Wound dressing: natural | Hyalofill (Anika) | Hyaluronic acid | Wound care and treatment | Absorb wound exudate, promote granulation tissue formulation, supports healing process | FDA (1999) |
| HemCon bandage (HemCon Medical Technologies) | Chitosan | Bandage | Provide hemostatsis and antibacterial barrier | FDA (2003) | |
| Regenecare wound gel (MPM Medical) | Collagen, aloe and sodium alginate with lidocaine | Wound filling | Management of skin wounds | FDA (2002) | |
| Purilon® Gel (Coloplast) | Sodium carboxymethylcellulose, calcium alginate | Wound filling | Dry and sloughy necrotic wounds as well as wounds with a mix of necrotic and granulated tissue | FDA (1997) | |
| Prontosan wound gel (B. Braun Medical) | Glycerol, hydroxyethyl cellulose with polyhexamethylene biguanide and undecylenamidopropyl betaine | Would gel | Management of ulcers, burns, partial and full thickness wounds, large surface area wounds and surgical incisions | FDA (2013) | |
| Algicell Ag calcium alginate dressing with antimicrobial silver (Integra Life Science) | Calcium alginate with silver | Wound dressing | Abrasions and minor lacerations, cuts, scalds and burns | FDA (2008) | |
| INTRASITE gel hydrogel wound dressing (Smith & Nephew Healthcare) | Modified carboxymethyl cellulose, propylene glycol | Wound dressing | Re‐hydrates necrotic tissue, facilitating autolytic debridement, loosen and absorb slough and exudate | FDA (1990) | |
| SoloSite Gel (Smith & Nephew Healthcare) | Sodium carboxymethylcellulose | Wound dressing | Minor burns, superficial lacerations, cuts and abrasions (partial thickness wounds), skin tears, venous ulcers (leg ulcers), surgical incisions, diabetic foot ulcers | FDA (1998) | |
| NU‐GEL™ (Systagenix) | Sodium alginate | Wound dressing | Chronic wounds | FDA (1998) | |
| ActivHeal Hydrogel (Advanced Medical Solutions) | Alginate | Wound dressing | Rehydrates dry necrotic wounds | FDA (2001) | |
| Surgical Silver Post Operative Dressing (Advanced Medical Solutions) | Alginate with silver | Wound dressing | Post‐operative surgical wounds | FDA (2018) | |
| Tissue augmentation: synthetic | IDEAL IMPLANT Saline‐filled Breast Implant (Ideal Implant) | Silicone | Implant | Breast implant | FDA (2014) |
| MENTOR® MemoryShape® Breast Implants (J&J MedTech) | Silicone | Implant | Breast implant | FDA (2013) | |
| Natrelle 410 Highly Cohesive Anatomically Shaped Silicone‐Filled Breast Implant (Allergan) | Silicone | Implant | Breast implant | FDA (2013) | |
| Sientra Silicone Gel Breast Implants (Sientra, Inc.) | Silicone | Implant | Breast implant | FDA (2013) | |
| Others | ReSure sealent (Ocular Therapeutix) | PEG | Topical | Intraoperative management of clear corneal incisions | FDA (2014) |
| Progel™ Pleural Air Leak Sealant (NEOMEND, Inc.) | Human serum albumin (HSA) and polyethylene glycol (PEG) | Surgical implant | Seal air leaks in both open and minimally invasive thoracic surgery | FDA (2010) | |
| Plenity® (Gelesis, Inc.) | Cellulose and citric acid | Oral | Overweight, obesity | FDA (2019) | |
Products that were included in the 2020 “Hydrogels in the clinic” review.
Abbreviations: CE, Conformité Européenne mark in the European Union; EMA, European Medicines Agency; FDA, Food and Drug Administration; HPMC, hydroxypropyl methyl cellulose; PEG, poly(ethylene glycol); PLGA, poly(lactic‐co‐glycolic acid).
3.1. New injectable hydrogel products
Approved injectable hydrogels cover multiple applications including facial correction, tissue repair and regeneration, drug delivery formulations/devices, among others. Consistent with our 2020 data, 1 injectable hydrogel‐based dermal fillers for facial correction remain a major area for approved products. These products are mainly formulated from natural polymers (e.g., HA and collagen) although synthetic materials (e.g., poly(lactic‐co‐glycolic acid) (PLGA), polymethylmethacrylate, carboxymethylcellulose) are also used in a few approved products. These products are typically injected to the dermis or subcutaneous space to correct winkles, folds, scars, or defective facial tissues. Since 2020, 6 new approvals have extended indications for an already‐approved product (RHA® Redensity, Restylane® Kysse, Restylane® Eyelight, Juvéderm® VOLBELLA XC, Juvéderm® VOLUX XC, SKINVIVE by Juvéderm®). These new approvals each indicate a previously approved hydrogel product for injection into a different facial site. For example, the approval of Restylane® Eyelight by the FDA in 2023 expanded the use of the Restylane® to include correction of infraorbital hollowing. Restylane® Eyelight was previously approved for correction of volume deficit, facial wrinkles and folds, and midface contour deficiency. 1
3.1.1. Tissue fusion, tissue repair, and tissue regeneration
Regenerative hydrogel products use injectable hydrogels to replace, repair or regenerate damaged, defective, or degenerated tissues. Approved hydrogel products or devices function as void fillers (e.g., Tactoset®, Grafton™ DBM), tissue fusion materials (e.g., INFUSE® bone graft, OP‐1® Putty), and visco‐supplements (SynoJoynt®, Arthrosamid®). Both synthetic (e.g., poloxamer, polyacrylamide, poly(ethylene glycol diacrylate) (PEGDA)), and natural polymers (e.g., HA, collagen, and chitosan) are extensively used in these productions with HA being the most common. While natural polymer‐based products afford advantages such as validated safety profiles and low immunogenicity, synthetic polymer‐based products may achieve better tunability by rational polymer design for desired hydrogel properties. Among them, one product that received FDA approval recently is Actifuse developed by Baxter. Actifuse is made from phase‐pure silicon‐substituted calcium phosphate in poloxamer 407 and was approved as bone void filler in spinal and orthopedic applications. 7
3.1.2. Drug delivery devices
Drug eluting hydrogel products use synthetic polymers. Two recently approved drug delivery formulations based on injectable synthetic hydrogels are Vantas and Jelmyto. Jelmyto, a hydrogel made from Pluronic F‐127, PEG‐400, and hydroxypropyl methyl cellulose (HPMC) for the delivery of mitomycin, received FDA approval in 2020 for the treatment of low‐grade upper tract urothelial cancer. 8 Vantas is a hydrogel depot comprised of hydroxyethyl methacrylate and hydroxypropyl methacrylate, crosslinked with trimethylolpropane trimethacrylate, which releases histrelin acetate for 12 months into the upper arm (following subcutaneous injection) for palliative treatment of advanced prostate cancer. Injectable hydrogels have also been approved for other applications and some of them include urinary incontinence (e.g., Bulkamid®), fecal incontinence (Solesta®), central precocious puberty (Supprelin LA®), protecting vulnerable tissues during radiotherapy (SpaceOAR®), among others.
3.2. Non‐injectable hydrogels
Unlike injectable hydrogels, non‐injectable hydrogels must be administered as a surgical implant, applied topically, or inserted non‐invasively with medical devices. Hydrogel dosage forms or formulations that are used for wound dressings, medical implants, and contact lens often use synthetic, non‐biodegradable, and/or mechanically robust materials (e.g., silicone, poloxamers, or carbomers) that are used less frequently for hydrogel injections. However, the approved non‐injectable hydrogels share some common applications with approved injectable hydrogels such as drug delivery, tissue repair, and tissue regeneration.
3.2.1. Bulk hydrogels as regenerative implants, tissue sealants, and delivery systems for pain management
Most bulk hydrogel products for implantation and drug delivery are made from synthetic polymers such as PEG and poly(vinyl alcohol) (PVA). Most approved non‐injectable hydrogels for tissue repair use natural polymers (collagen in particular) as the backbone material. These hydrogels are typically implanted or inserted, rather than injected, for repair of diabetic foot ulcer (e.g., Apligraf®), tendon and ligamentous tissue (e.g., GRAFTJACKET Now, Permaco, TissueMend®, Permacol®), rotator cuff repair (Zimmer® Collagen Repair Patch), and other applications. DuraSeal is an approved non‐injectable hydrogel derived from a synthetic polymer for tissue repair. DuraSeal is comprised of PEG ester, trilysine amine, and decahydrated sodium borate. DuraSeal is indicated for the prevention of cerebrospinal fluid leakage after cranial and spinal surgery. 9 Hydrogels have further been used to promote regeneration of diverse tissues such as bone, tendon, ligament, and for the treatment of osteoarthritis. For example, a recent study showed that intradiscal injection of ReGelTec's Hydrafil hydrogel relieved chronic lower back pain caused by degenerative disc disease. Patients' pain level decreased from 7.1 to 2.0 (on a scale of 10) and they also experienced greatly improved physical function. 10 These findings led to designation as an FDA Breakthrough Device.
3.2.2. Bulk hydrogel contact lenses
Most of the approved hydrogel‐based contact lenses or ocular formulations use synthetic polymer‐based hydrogel such as silicone, nelfilcon A, carbomer, PEG, PVA, or poloxamer, although natural polymers such as HA and collamer are also used in some products. These products cover multiple ocular indications. Noted examples include astigmatism, myopia, hyperopia, drug eye, uveitis, macular edema, and glaucoma. Abiliti Overnight Therapeutic Lenses, Precision 7 family, and EVO TICL are three examples of recently approved hydrogel‐based contact lenses. For instance, the Precision 7 family including Precision 7, Precision 7 for Astigmatism, Precision 7 Multifocal, and Precision7 Multifocal Toric are Senofilcon A based soft contact lenses that are indicated for ocular indications including myopia, hyperopia, and astigmatism.
3.2.3. Hydrogel wound dressings
Hydrogel wound dressings are another important class of approved non‐injectable hydrogel products, among which both synthetic and natural hydrogels are extensively used. Skin is the largest organ, provides an important barrier, and serves in immune functions. 11 Compared to other organs, skin is relatively accessible and can typically be accessed in a minimally invasive manner. While we already described hydrogels for aesthetic dermal applications, other hydrogel products have been formulated for topical application and promotion of wound healing. For example, Apligraf® is an FDA‐approved product to treat chronic wounds such as diabetic foot ulcers and venous leg ulcers. 12 This product can also be readily reapplied as needed. These approved wound dressing products are applied to the wound through diverse formats such as bandage, hydrogel sheet, gauze, and would‐filling gel to promote wound healing.
3.2.4. Bulk hydrogel‐based surgical products
Additional surgical applications utilize approved hydrogel products. Silicone‐based hydrogels are used in plastic surgery, surgical reconstruction, and as tissue sealants.
4. CLINICAL TRIALS
A comprehensive analysis of current hydrogel clinical trials was performed in the ClinicalTrials.gov database. Active, recruiting, and enrolling trials were included, with completed, terminated, suspended, or withdrawn trials excluded. An initial collection of potentially admissible clinical trials was collected by searching for “hydrogel” in trial intervention (80 trials), “hydrogel” as a search term (89 trials), “gel” as an intervention (518 trials) and “extracellular matrix” as an intervention (24 trials). Once duplicate returns were removed, our dataset contained 632 active “gel”, “hydrogel”, or “ECM” trials. The dataset was refined further by excluding observational or non‐interventional clinical trials, excluding trials where the hydrogel was not an active component of the intervention (e.g., where a gel pad was used as a dressing in a medical device trial), and excluding any trial where the intervention did not meet the working definition of a hydrogel (i.e., crosslinked polymers swollen in an aqueous solution). The resulting dataset contained 210 unique hydrogel clinical trials. A content analysis was performed, where each trial was categorized by the hydrogel form (i.e., bulk material, patch or dressing, particulate suspension, lens, or coil), disease indicated, material origin (i.e., natural, synthetic, both, or unknown), and whether an active agent was delivered. Product names, biomaterial compositions, and specific drugs delivered were also noted. A summary of these data is presented in Figure 1.
FIGURE 1.
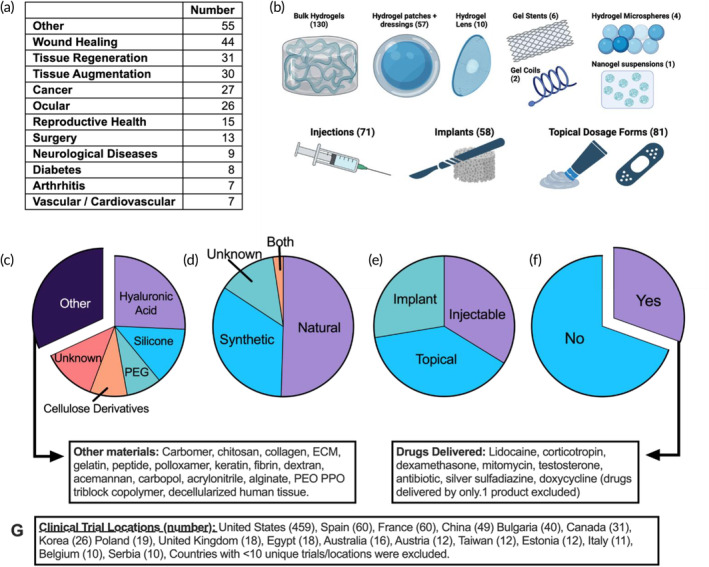
Overview of current hydrogel clinical trials. (a) Table summary of the cumulative number of clinical trials per indication. (b) Summary of hydrogel dosage forms, with the cumulative number of trials and a representative depiction. Graphic generated using BioRender. (c–f) Part‐of‐whole analysis summarizing the biomaterials, material origin, dosing method, and drug delivery of hydrogel clinical trials. (g) Summary of global clinical trial sites. The total number of sites is much more than 210, as single clinical trials often have many trial sites/locations. Data collected from ClinicalTrials.gov and are current as of June 20, 2023.
4.1. Clinical trial overview and update
Tissue regeneration, soft tissue augmentation, cancer, urinary incontinence, and related indications have persisted as leading applications for hydrogel products, with approximately 100 unique current clinical trials and a similar number of unique products. Our expanded scope for data collection led to identification of wound healing products as the most common application for hydrogel products in current clinical trial. Our analysis also revealed new biomaterials (i.e., no active trials with the material composition in 2020) and new applications (i.e., new clinical trial indications for hydrogel therapeutics that have no associated approved products and were not captured in our 2020 analysis).
4.2. An update on injectable hydrogel clinical trials
Four injectable hydrogel products that were captured in our 2020 analysis have ongoing clinical trials in 2024. (Algisyl‐LVR for intra‐myocardial injection after heart failure, 13 , 14 TraceIT for cancer imaging and tissue spacing in radiation therapy, 15 SpaceOAR for tissue spacing in radiation therapy, 16 , 17 and Bulkamid for transurethral injection to treat stress urinary incontinence 18 , 19 ), are approved by FDA or EMA. One of those products (SpaceOAR) has six current/active clinical trials. PAAG‐OA (Contura), 20 an intra‐articular injection of poly(acrylamide) hydrogel, is still being evaluated in two new trials (NCT04179552 and NCT04045431) in Denmark for knee osteoarthritis. Gelstix, an injectable poly(acrylonitrile) hydrogel for treatment of lumbar intervertebral disc degeneration, 21 is still being evaluated in NCT02763956 in Switzerland and the Netherlands. The remaining 66 current injectable hydrogel trials involve both new and previously approved products. Nearly half of those products are comprised of hyaluronic acid (31 unique trials, including the following products: MateRegen, 22 , 23 Hyalobarrier, 24 HAmonyCa, 25 KD Intra‐Articular gel, IPN‐21‐SENSE, Juvéderm, 26 Sestylane, GAL 1906, Stylage, Auralya, I.SPACE, Belotero, Papilocare), or PEG (eight unique trials, including products Juveena, ReSpace, LifePearl, 27 Instylla HES, 28 and BioSentry BioSeal 29 ). Noteworthy new products, which explore new hydrogel biomaterials or new hydrogel indications will be described in a later section.
4.3. Summary of non‐injectable hydrogel clinical trials
Our expanded clinical trial search yielded 139 new trials involving topical hydrogel application (81 trials) or non‐injectable hydrogel implants (58 trials). Representative trials are summarized in Table 2. Topical application of wound dressings, hydrogel contact lenses, and transdermal drug delivery systems are being tested for their treatment of several indications. Skin wounds treated with hydrogel therapeutics include radiation dermatitis, diabetic ulcers, and burns. 30 , 31 Biomaterials for topical dosage forms include synthetic carbomers, carboxymethylcellulose, chitosan, keratin, and poloxamer, in addition to the backbone polymers that are common across all hydrogel applications (i.e., HA, PEG, and silicone). Silicone continues to be especially common for application in hydrogel contact lenses, 32 , 33 with several clinical trials and approved products. Hydrogel implants are being applied for regenerative medicine, tissue augmentation, treatment of dental/periodontal disease, and prevention of postsurgical adhesions. Backbone hydrogel materials included a variety of biomaterials, including silicone hydrogels, non‐HA polysaccharide hydrogels, and ECM hydrogels, which are uncommon backbone polymers for both injectable hydrogels and current approved products. Six gel stent trials (XEN stent) 34 are currently evaluating outcomes in the treatment of glaucoma.
TABLE 2.
Summary of representative recruiting, enrolling, active clinical trials involving topical hydrogels, hydrogel contact lenses, and hydrogel implants.
| Name (Company) | Indication | Hydrogel formulation | Drug delivered? | NCT number (Trial Phase) |
|---|---|---|---|---|
| Representative products—hydrogel implants in clinical trial | ||||
| BEAR Implant (Miach Orthopedics) | Anterior cruciate ligament injuries | Bovine collagen implant | No | NCT05398341 (not given) |
| Myriad Matrix, Myriad Morcells (Aroa Biosurgery Ltd.) | Abdominal Wound Dehiscence|Necrotizing Soft Tissue Infection|Lower Extremity Wound|Pilonidal Sinus|Anal Fistula|Hidradenitis Suppurativa|Pressure Injury | ECM implant | No | NCT05243966 (not given) |
| HANBIO BarriGel (HAN Biomedical Inc.) | Adhesion|Thyroid Diseases | Hyaluronic acid implant | No | NCT05036525 (NA) |
| BrachyGel (Brachy Foam, LLC) | Cervical cancer | PEG implant | No | NCT04499521 (NA) |
| DEXTENZA (Ocular Therapeutix, Inc.) | Vitreoretinal surgery, pain, inflammation | PEG implant | Yes, dexamethasone | NCT04371445 (Ph 4) and NCT04200651 (Ph 4) |
| AxoGuard (Axogen Corp.) | Symptomatic neuroma, chronic nerve pain | Porcine ECM implant | No | NCT03940963 (NA) and NCT04865679 (NA) |
| ESTYME (Symatese Aesthetics) | Bilateral breast augmentation | Silicone implant | No | NCT05336526 (NA) |
| Silimed (Silimed Industria de Implantes Ltda.) | Breast implant | Silicone implant | No | NCT03356132 (not given) |
| Motiva Implant (Motiva USA LLC) | Breast implant | Silicone implant | No | NCT03579901 (NA) |
| Representative products—topical hydrogels | ||||
| PTX‐022 (Palvella Therapeutics, Inc.) | Pachyonychia congenita, basal cell carcinomas in Gorlin syndrome patients | Topical carbomer 940 | No | NCT05643872 (Ph 3) and NCT04893486 (Ph 2) |
| TCP‐25 (Xinnate AB) | Blister skin wound | Topical hydroxyethylcellulose | No | NCT05378997 (Ph 1) |
| Catasyn (Synedgen, Inc.) | Superficial partial thickness burn | Topical hydroxypropylcellulose | No | NCT04601532 (Ph 4) |
| StrataMGT (Strataphama AG) | Genitourinary syndrome of menopause | Topical silicone | No | NCT05672901 (NA) |
| StrataXRT (Stratapharma AG) | Radiation dermatitis | Topical silicone | No | NCT05553392 (NA) |
| Suprasorb (Lohmann & Rauscher) | Ulcer | Topical alginate | No | NCT05646121 (not given) |
| RadiaAce (AceTech) | Breast cancer radiation dermatitis | Topical acemannan | No | NCT04481802 (NA) |
| QY211 (E‐nitiate Biopharmaceuticals Co., Ltd. | Atopic dermatitis | Topical carbomer 947 | No | NCT05843422 (Ph 1) |
| NanoDOX (NanoSHIFT LLC) | Wounds and injury | Topical carboxymethylcellulose | Yes, doxycycline | NCT05411484 (Ph 2) |
| NanoSALV (NanoTess, Inc.) | Ulcer | Topical cellulose | No | NCT05619237 (NA) |
| SynePure (Synedgen, Inc.) | Burn wounds | Topical chitosan | Yes, silver sulfadiazine | NCT05877638 (NA) |
| TTAX01 (Tissue Tech, Inc.) | Diabetic foot infection, non‐healing wound | Topical decellularized human placental umbilical cord tissue | No | NCT04450693 (Ph 3) and NCT04176120 (Ph 3) |
| Restrata, Apligraf (Acera Surgical, Inc.) | Diabetic foot ulcer, venous leg ulcer | Topical electrospun ECM | No | NCT04927702 (NA) |
| Juvia (FEMPHARMA Kft.) | Vaginal yeast infections | Topical hydroxyethylcellulose | Yes, zinc | NCT05895162 (NA) |
| KeraStat (KeraNetics, LLC) | Partial‐thickness burn | Topical keratin | No | NCT03564795 (NA) |
| BIAKOS (SeranaGropu, Inc.) | Wound of skin | Topical poloxamer407 | Yes, polyaminopropyl biguanide | NCT05107050 (NA) |
| Representative product—hydrogel stent | ||||
| XEN45 (Allergan/AbbVie) | Open‐angle glaucoma | Porcine collagen‐derived gelatin hydrogel stent | No | NCT05411198 (Ph 3) |
| Representative product—hydrogel coil | ||||
| HydroSoft (Microvention‐Terumo, Inc.) | Ruptured aneurysm | Vinyl polymer hydrogel coil | No | NCT03252314 (not given) |
Note: Trials are organized first by material form, then biomaterial composition.
4.4. New hydrogel biomaterials in active clinical trials
Our analysis identified three noteworthy categories of new hydrogel clinical trials, which are summarized in Table 3. The grouping includes five clinical trials (NCT04379700, NCT04951479, NCT05268718, NCT04595266, and NCT05051332) which are evaluating some of the first hydrogel nanogel or hydrogel microsphere suspensions for the treatment of diverse indications (e.g., osteoarthritis, ulcerative keratitis, metastatic cancer, and cartilage degeneration). The second are nine active trials involving new hydrogel biologic or hydrogel‐cell combination products (Products: SygeLIX, TumoCure, BioVAT, Placental Mesenchymal Stem Cells on Dural ECM, ALLO‐ACS‐DFU, DBI‐001, DBI‐002, TTAX01, and ArthoFLEX). The final grouping highlights clinical trials evaluating new hydrogel materials, which have been designed to treat indications such as chronic rhinosinusitis, ruptured aneurysm, bacterial vaginoses, periodontal disease, bladder cancer, and more.
TABLE 3.
Summary of recruiting, enrolling, active clinical trials using novel hydrogel biomaterials or dosage forms.
| Name (sponsor company or university) | Indication | Route of administration | Hydrogel formulation | Drug delivered | ClinicalTrials.gov identifier (trial phase) |
|---|---|---|---|---|---|
| First nanogel/microgel based delivery systems as combination products | |||||
| Embozene microspheres (NYU Langone Health) | Osteoarthritis | Intraarticular injection | Polyzene‐F microspheres | No | NCT04379700 (NA) |
| OptiSphere (University of Pittsburgh) | Knee osteoarthritis | Intraarterial injection | Microspheres (unknown material) | No | NCT04951479 (NA) |
| Hollow gold and silver alloy cuprous oxide shell nano‐shell hydrogel (Zhejang University) | Ulcerative keratitis, antibiotic resistance, photothermal therapy | Topical application | Nanogel suspension | No | NCT05268718 (early Ph I) |
| LifePearl (Grupo Espanol Multidisciplinario del Cancer Digestivo) | Metastatic colorectal cancer, liver metastasis | Intraarterial injection | PEG microgel suspension | Yes, irinotecan | NCT04595266 (Ph II) |
| CartiLife (Biosolution Co., Ltd.) | Articular cartilage defect, articular cartilage degeneration | Intraarticular injection | Fibrin microsphere | Yes, chondrocytes | NCT05051332 (Ph III) |
| Gel biologics + cell combination products | |||||
| SygeLIX‐F, SygeLIX‐G (TBF Genie Tissulaire) | Anal fistula | Implant via internal orifice | Bulk Wharton's Jelly gel plug | Yes, stem cells | NCT05638139 (Ph I) |
| TumoCure (IntraGel Therapeutics) | Head and neck cancer | Intratumoral injection | Bulk polymeric gel | Yes, cisplatin | NCT05200650 (Ph I) |
| BioVAT (University Medical Center Coettingen) | Heart failure | Implant in ventricular myocardium | Bulk collagen hydrogel with iPSC‐derived cardiomyocytes | Yes, cardiomyocytes | NCT04396899 (Ph I, Ph II) |
| Placental mesenchymal stem cells on dural ECM (California Institute for Regenerative Medicine) | Myelomeningocele | Topical application | Bulk dural graft ECM hydrogel | Yes, mesenchymal stem cells | NCT04652908 (Ph I, Ph II) |
| ALLO‐ACS‐DFU (Anterogen Co., Ltd.) | Diabetic foot ulcer | Topical application | Hydrogel SHEET dressing | Yes, allogenic mesenchymal stem cells | NCT03754465 (Ph II) |
| DBI‐001, DBI‐002 (DermBiont, Inc.) | Tinea pedis | Topical application | Unknown aqueous gel | Yes, microbe | NCT05493488 (Ph II) |
| TTAX01 (Tissue Tech Inc.) | Non‐healing diabetic foot ulcer | Topical application | Decellularized human placental umbilical cord tissue | No | NCT04450693 (Ph III) |
| TTAX01 (Tissue Tech Inc.) | Non‐healing diabetic foot ulcer | Topical application | Decellularized human placental umbilical cord tissue | No | NCT04176120 (Ph III) |
| ArthoFLEX ECM scaffold (Cleveland Clinic) | Rotator cuff tear | Implant via arthroscopic procedure | Bulk ECM hydrogel | No | NCT03551509 (Ph IV) |
| New polymers/materials (fewer than two trials and no approved products) | |||||
| Chitodex Gel (St. Louis University) | Chronic rhinosinusitis | Topical application in nasal cavity | Chitosan and dextran | No | NCT05083741 (not given) |
| CartRevive (Hy2Care BV, Avania, UMC Utrecht) | Cartilage damage | Intraoperative injection in knee | Dextran + hyaluronic acid | No | NCT05186935 (NA) |
| Multi‐Gyn ActiGel (BioClin BV, Avania, Karo Pharma AB) | Bacterial vaginoses | Topical application to vagina | Galactoarabinan polyglucoronic acid crosspolymer | No | NCT04807842 (NA) |
| Aquacryl (Ain Shams Maternity Hospital) | Early abortion | Implant in cervical canal | Poly(acrylonitrile) | No | NCT05147857 (NA) |
| Condrotide® (Mastelli S.r.I, Latis S.r.I.) | Meniscus tear, meniscus lesion | Intraarticular and intrameniscal injection | Polynucleotide | No | NCT05322005 (NA) |
| Plenhyage® (I.R.A. Istituto Ricerche Applicate S.p.A.|Opera CRO, a TIGERMED Group Company) | Cicatrix, lipodystrophy, plaque | Intradermal injection | Polynucleotides (PDRN) of animal origin (fish) | No | NCT05239117 (NA) |
| Emdogain® FL (Aristotle University of Thessaloniki) | Periodontal Disease, AVDC Stage 3 and 4 | Periodontal implant | Propylene glycol alginate and Porcine enamel matrix derivative | No | NCT05541614 (NA) |
| SMI‐01 (Sofregen Medical, Inc., Symbio, LLC). | Nasolabial fold, cheek augmentation | Intradermal injection | Silk | No | NCT04534660 (NA) |
| Particulate xenograft erythropoietin gel (Ain Shams University) | Periodontal bone loss | Periodontal implant | Erythropoietin and carboxymethylcellulose | Yes, particulate xenograft | NCT05360511 (Early Ph I) |
| PRO‐165 (Laboratorios Sophia S.A de C.V. | Dry eye syndromes | Topical application to eye | Chondroitin sulfate and hyaluronic acid | No | NCT03697876 (Ph I) |
| UGN‐102 (UroGen Pharma Ltd.) | Bladder cancer, urothelial carcinoma | Intravesicular injection (into bladder via catheter) | PEO/PPO triblock copolymer | Yes, mitomycin | NCT05243550 (Ph III) |
| UGN‐102 (UroGen Pharma Ltd.) | Bladder cancer, urothelial carcinoma | Intravesicular injection (into bladder via catheter) | PEO/PPO triblock copolymer | Yes, mitomycin | NCT05136898 (Ph III) |
| Hydrotac (Centre Francois Baclesse, Ministry of Health, France) | Head and neck cancer | Topical application to skin | Polyurethane | No | NCT01520701 (Ph III) |
| Chitosan‐based erythropoietin gel (Ain Shams University) | Erythropoietin recession | Topical application to gingiva | Chitosan and beta glycerophosphate | Yes, erythropoetin | NCT05683782 (Ph 4) |
Note: Trials are organized by their category of technical novelty.
4.5. New indications for hydrogel therapy in active clinical trial
Our research identified a final set of noteworthy active clinical trials, where hydrogel therapeutics (approved and new) are being evaluated for new indications. These clinical trials are summarized in Table 4. These indications, which non‐exhaustively include osteoarthritis of joints other than the knee, esophageal and colorectal diseases, surgically acquired infection, nerve pain, and infertility currently lack approved hydrogel products. Most of these trials are evaluating common backbone polymers (i.e., HA, PEG, carbomer, and ECM), formulated into first‐in‐class hydrogel products. Some of these products (e.g., ADAM system, a male birth control device developed by Contraline Inc.) 35 propose truly emerging therapeutics, as they offer a solution for a medical problem or disease indications with no approved biomaterial or pharmacological products.
TABLE 4.
Summary of recruiting, enrolling, and active clinical trials proposing new disease indications for hydrogel‐based dosage forms, delivery systems, or therapeutics.
| Name (sponsor company or university) | Indication | Route of administration | Hydrogel formulation | Drug delivered | ClinicalTrials.gov Identifier (Trial Phase) |
|---|---|---|---|---|---|
| KD Intra‐Articular® gel (Procare Health Iberia S.L.) | Hip, thumb, or ankle osteoarthritis | Intraarticular injection | Hyaluronic acid | No | NCT05275244 (not given) |
| Purastat (AdventHealth) | Esophageal stricture | Topical application to GI lesions via endoscope | Synthetic peptide | No | NCT05581173 (not given) |
| Bridge‐Enhanced ACL Restoration (BEAR®) (Miach Orthopedics) | Anterior cruciate ligament injuries | Surgical implant | Bovine collagen | No | NCT05398341 (not given) |
| ORISE gel (Portsmouth Hospitals NHS Trust, etc.) | Colorectal polyp | Submucosal injection during endoscopy | Poloxamer | No | NCT04886609 (not given) |
| Condrotide (Mastelli S.r.I, Latis S.r.I.) | Meniscus tear, meniscus lesion | Intraarticular and intrameniscal injection | Polynucleotide | No | NCT05322005 (NA) |
| DAC‐AE (Centre Hospitalier Universitaire de Saint Etienne, Ministry of Health, France) | Hip prosthesis infection | Surgical implant | Hyaluronic acid and poly(lactic acid) | Yes, antibiotic | NCT04251377 (NA) |
| DAC, Novagenit SRL (Centre Hospitalier Universitaire de Saint Etienne, Ministry of Health, France) | Hip prosthesis infection | Surgical implant | Hyaluronic acid, poly‐lactic acid | Yes, antibiotic | NCT04251377 (NA) |
| Papilocare (Procare Health Iberia S.L., Adkoma Health Research) | Human papilloma virus, human papilloma virus infection, cervix lesion | Intravaginal injection via cannula | Hyaluronic acid, beta glucans | No | NCT04199078 (NA) |
| Axoguard Nerve Cap (Axogen Corp.) | Symptomatic neuroma, amputation, chronic nerve pain | Surgical implant | Porcine ECM | No | NCT04865679 (NA) |
| Axoguard Nerve Cap (Axogen Corp.) | Symptomatic neuroma, Morton's neuroma, chronic nerve pain | Surgical implant | Porcine ECM | No | NCT03940963 (NA) |
| GelStix (Ospedale Regionale di Lugano, Rijnstate Ziekenhuis, Arnhem, the Netherlands) | Degeneration of lumbar intervertebral disc | Intradiscal injection | Hydrolysed poly(acrylonitrile) | No | NCT02763956 (NA) |
| MectaShield (Medacta International SA) | Arthritis, traumatic arthritis, avascular necrosis | Surgical implant | Unknown | Yes, antibiotic | NCT05679232 (NA) |
| JointRep (Oligo Medic Pty Ltd. Mobius Medical Pty Ltd.) | Articular cartilage defect | Intraarticular injection | Chitosan | No | NCT04840147 (NA) |
| ADAM system (Contraline Inc.) | Azoospermia, oligospermia | Injection into vas deferens | Unknown | No | NCT05134428 (NA) |
| MateRegen (Chinese University of Hong Hong) | First trimester abortion, surgical abortion, miscarriage with afibrinogenemia | Intrauterine injection | Hyaluronic acid | No | NCT05360186 (NA) |
| Hyalobarrier Gel (Ghent University Hospital, etc.) | Infertility, uterine polyp uterine myoma, uterine adhesion, hysteroscopy, uterine septum | Intrauterine injection | Hyaluronic acid | No | NCT03880435 (NA) |
| Hyalobarrier Gel (Ghent University Hospital) | Myoma | Intrauterine injection | Hyaluronic acid | No | NCT05683041 (NA) |
| Hyalobarrier Gel (SciVision Biotech Inc) | Tissue adhesion in gynecologic surgery | Intrapelvic implant | Hyaluronic acid | No | NCT04063085 (NA) |
| Juvia (Fempharma Kft.) | Vaginal yeast infections | Topical application to vagina | Hydroxyethylcellulose | Yes, zinc | NCT05895162 (NA) |
| StrataMGT (Stratpharma AG) | Genitourinary syndrome of menopause | Topical application to vagina | Silicone | No | NCT05672901 (NA) |
| VersaWrap (University of Colorado, Denver) | Distal radius fracture, tendon rupture | Surgical Implant | Hyaluronic acid and alginate | No | NCT04976335 (NA) |
| HANBIO BarriGel (HAN Biomedical Inc.) | Adhesion, thyroid diseases | Surgical Implant | Hyaluronic acid | No | NCT05036525 (NA) |
| Embrace Hydrogel Embolic System (HES) (Instylla, Inc.) | Arterial bleeding in solid organs and peripheral arteries | Intraarterial injection | PEG | No | NCT05364502 (NA) |
| Hydrocoil (Seoul National University Hospital, etc.) | Ruptured aneurysm, intracranial aneurysm | Intracranial implant in Aneurysm | Unknown | No | NCT04988503 (NA) |
| ATX01 (Algo Therapeutix) | Chemotherapy‐induced peripheral neuropathy | Transdermal delivery | Unknown | Yes, amitriptyline hydrochloride | NCT05593614 (Ph II) |
| Androgel (University Hospital, Clermont‐Ferrand) | Hypermetabolism in ICU | Transdermal delivery | Carbomer 980 | Yes, testosterone | NCT03678233 (Ph II) |
| Androgel (VA Office of Research and Development) | Type 2 diabetes mellitus, hypogonadism | Transdermal delivery | Carbomer 980 | Yes, testosterone | NCT03887936 (Ph IV) |
| Juveena (Rejoini Inc.) | Intrauterine adhesion | Intrauterine injection | PEG | No | NCT05394662 (Ph III) |
| FORTESTA (Endo Pharmaceuticals) | Male hypogonadism, hypogonadotropic hypogonadism | Transdermal delivery | Carbomer | Yes, testosterone | NCT04456296 (Ph IV) |
Note: Trials are organized by phase.
5. CONCLUSION
Hydrogel materials are exciting candidates for therapeutics due to their ability to mimic native tissue environments and deliver a variety of therapeutic payloads. Our analysis indicates that hydrogels have been successfully translated as more than 100 medical products, for a variety of aesthetic, tissue repair, wound healing, tissue support, cancer, and ophthalmic applications. Emerging applications for therapeutic hydrogels include hydrogel coils for aneurysm treatment, hydrogel stents for glaucoma, new hydrogel wound dressings or patches for burns, diabetic ulcers, and radiation‐induced skin damage. New injectable hydrogels are being evaluated for application in pain management, regeneration of non‐musculoskeletal tissues, treatment of infection, female reproductive health, and male birth control. New non‐injectable hydrogels have been formulated as well, as coatings on medical implants, as bulk material implants, and in other forms. Depending on the nature of a new hydrogel device, its intended use, and its substantial equivalence to previously approved products, these new products may be classified as a class I, II, or III device. It is noteworthy that current hydrogel devices have received a range of classifications from the FDA, from silicone hydrogels and hydrogel burn dressings (class I device), to injectable hydrogel spacers for rectal radiotherapy (class II device) and wound dressings containing drugs and biologics (currently unclassified and pre‐amendment). While these translational challenges to hydrogels and hydrogel combination products persist, the presented data indicate that alternate hydrogel dosage forms (e.g., topical dressings, implants, stents, coils, microspheres, and nanogel suspensions) and new materials (e.g., peptide gels, nucleotide gels, and new synthetic polymers) are enabling a wide range of therapeutic hydrogel applications.
AUTHOR CONTRIBUTIONS
John R. Clegg: Conceptualization; formal analysis; methodology; writing – original draft; writing – review and editing. Kolade Adebowale: Conceptualization; formal analysis; methodology; writing – original draft; writing – review and editing. Zongmin Zhao: Conceptualization; formal analysis; methodology; writing – original draft; writing – review and editing. Samir Mitragotri: Conceptualization; supervision; writing – review and editing.
PEER REVIEW
The peer review history for this article is available at https://www.webofscience.com/api/gateway/wos/peer-review/10.1002/btm2.10680.
ACKNOWLEDGMENTS
S. M. and K. A. acknowledge support from the John A Paulson School of Engineering & Applied Sciences, Harvard University and Wyss Institute. K. A. acknowledges support from the National Science Foundation MPS‐Ascend Postdoctoral Fellowship, Award No. (FAIN): 2138064, and the National Institute of General Medical Sciences of the National Institutes of Health under award number FAIN# K99GM151568. J. R. C. acknowledges that research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under award number R35GM150970. J. R. C. also acknowledges that this publication is supported by Institutional Research Grant number 134128‐IRG‐19‐142‐01 from the American Cancer Society. Z. Z. acknowledge support from Vahlteich Award and Startup fund from the College of Pharmacy, University of Illinois Chicago. S. M. is a shareholder of, board member of and consultant to Fount Bio. S. M. is an inventor of patents related to hydrogels.
Clegg JR, Adebowale K, Zhao Z, Mitragotri S. Hydrogels in the clinic: An update. Bioeng Transl Med. 2024;9(6):e10680. doi: 10.1002/btm2.10680
Contributor Information
John R. Clegg, Email: clegg@ou.edu.
Zongmin Zhao, Email: zhaozm@uic.edu.
Samir Mitragotri, Email: mitragotri@seas.harvard.edu.
DATA AVAILABILITY STATEMENT
All data are available in the main text.
REFERENCES
- 1. Mandal A, Clegg JR, Anselmo AC, Mitragotri S. Hydrogels in the clinic. Bioeng Transl Med. 2020;5(2):e10158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Correa S, Grosskopf AK, Lopez Hernandez H, et al. Translational applications of hydrogels. Chem Rev. 2021;121(18):11385‐11457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Richbourg NR, Peppas NA. The swollen polymer network hypothesis: quantitative models of hydrogel swelling, stiffness, and solute transport. Prog Polym Sci. 2020;105:101243. [Google Scholar]
- 4. Clegg JR, Wagner AM, Shin SR, Hassan S, Khademhosseini A, Peppas NA. Modular fabrication of intelligent material–tissue interfaces for bioinspired and biomimetic devices. Prog Mater Sci. 2019;106:100589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Koetting MC, Peters JT, Steichen SD, Peppas NA. Stimulus‐responsive hydrogels: theory, modern advances, and applications. Mater Sci Eng R Rep. 2015;93:1‐49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhang YS, Khademhosseini A. Advances in engineering hydrogels. Science. 2017;356(6337):eaaf3627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ovrebo O, Perale G, Wojciechowski JP, et al. Design and clinical application of injectable hydrogels for musculoskeletal therapy. Bioeng Transl Med. 2022;7(2):e10295. doi: 10.1002/btm2.10295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rafael D, Melendres MMR, Andrade F, et al. Thermo‐responsive hydrogels for cancer local therapy: challenges and state‐of‐art. Int J Pharm. 2021;606:120954. doi: 10.1016/j.ijpharm.2021.120954 [DOI] [PubMed] [Google Scholar]
- 9. Tarafder S, Park GY, Felix J, Lee CH. Bioadhesives for musculoskeletal tissue regeneration. Acta Biomater. 2020;117:77‐92. doi: 10.1016/j.actbio.2020.09.050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. ReGelTec's Hydrafil injectable hydrogel studied to treat low back pain caused by degenerative disc disease. Endovascular today. IEEE; 2024. [Google Scholar]
- 11. Abdallah F, Mijouin L, Pichon C. Skin immune landscape: inside and outside the organism. Mediators Inflamm. 2017;4:1‐17. doi: 10.1155/2017/5095293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kathawala MH, Ng WL, Liu D, et al. Healing of chronic wounds: an update of recent developments and future possibilities. Tissue Eng Part B‐Rev. 2019;25(5):429‐444. doi: 10.1089/ten.teb.2019.0019 [DOI] [PubMed] [Google Scholar]
- 13. Lee RJ, Hinson A, Bauernschmitt R, et al. The feasibility and safety of Algisyl‐LVR™ as a method of left ventricular augmentation in patients with dilated cardiomyopathy: initial first in man clinical results. Int J Cardiol. 2015;199:18‐24. [DOI] [PubMed] [Google Scholar]
- 14. Cattelan G, Guerrero Gerbolés A, Foresti R, et al. Alginate formulations: current developments in the race for hydrogel‐based cardiac regeneration. Front Bioeng Biotechnol. 2020;8:414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Greer M, Schaub SK, Bowen SR, et al. TraceIT: a prospective pilot study of a temporary intravesical fiducial marker for bladder cancer radiation therapy. American Society of Clinical Oncology; 2021. [Google Scholar]
- 16. Hall WA, Tree AC, Dearnaley D, et al. Considering benefit and risk before routinely recommending SpaceOAR. Lancet Oncol. 2021;22(1):11‐13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Armstrong N, Bahl A, Pinkawa M, et al. SpaceOAR hydrogel spacer for reducing radiation toxicity during radiotherapy for prostate cancer. A systematic review. Urology. 2021;156:e74‐e85. [DOI] [PubMed] [Google Scholar]
- 18. Kasi AD, Pergialiotis V, Perrea DN, Khunda A, Doumouchtsis SK. Polyacrylamide hydrogel (Bulkamid®) for stress urinary incontinence in women: a systematic review of the literature. Int Urogynecol J. 2016;27:367‐375. [DOI] [PubMed] [Google Scholar]
- 19. Brosche T, Kuhn A, Lobodasch K, Sokol ER. Seven‐year efficacy and safety outcomes of Bulkamid for the treatment of stress urinary incontinence. Neurourol Urodyn. 2021;40(1):502‐508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bliddal H, Overgaard A, Hartkopp A, Beier J, Conaghan P, Henriksen M. Polyacrylamide hydrogel injection for knee osteoarthritis: a 6 months prospective study. J Orthop Res Ther. 2021;6(2):S278. [Google Scholar]
- 21. Ceylan A, Aşik İ, Özgencil GE, Erken B. Clinical results of intradiscal hydrogel administration (GelStix) in lumbar degenerative disc disease. Turk J Med Sci. 2019;49(6):1634‐1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Liu Y‐R, Liu B, Yang B‐P, Lan Y, Chi Y‐G. Efficacy of hyaluronic acid on the prevention of intrauterine adhesion and the improvement of fertility: a meta‐analysis of randomized trials. Complement Ther Clin Pract. 2022;47:101575. [DOI] [PubMed] [Google Scholar]
- 23. Zhou Q, Shi X, Saravelos S, et al. Auto–cross‐linked hyaluronic acid gel for prevention of intrauterine adhesions after hysteroscopic adhesiolysis: a randomized controlled trial. J Minim Invasive Gynecol. 2021;28(2):307‐313. [DOI] [PubMed] [Google Scholar]
- 24. Esber S, Etrusco A, Laganà AS, et al. Clinical outcomes after the use of antiadhesive agents in laparoscopic reproductive surgery. Gynecol Obstet Invest. 2023;88(6):325‐335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Urdiales‐Gálvez F, Braz A, Cavallini M. Facial rejuvenation with the new hybrid filler HArmonyCa™: clinical and aesthetic outcomes assessed by 2D and 3D photographs, ultrasound, and elastography. J Cosmet Dermatol. 2023;22:2186‐2197. [DOI] [PubMed] [Google Scholar]
- 26. Kapoor KM, Saputra DI, Porter CE, et al. Treating aging changes of facial anatomical layers with hyaluronic acid fillers. Clin Cosmet Investig Dermatol. 2021;14:1105‐1118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. de Baere T, Plotkin S, Yu R, Sutter A, Wu Y, Cruise GM. An in vitro evaluation of four types of drug‐eluting microspheres loaded with doxorubicin. J Vasc Interv Radiol. 2016;27(9):1425‐1431. [DOI] [PubMed] [Google Scholar]
- 28. Goh GS, Goodwin MD, Huang J‐F, Kavnoudias H, Holden A. A pilot first‐in‐human study of embrace, a polyethylene glycol‐based liquid embolic agent, in the embolization of malignant and benign hypervascular tumors. J Vasc Interv Radiol. 2022;33(6):660‐667. [DOI] [PubMed] [Google Scholar]
- 29. Jungmann MA, Recalde Phillips S, Touchet TJ, et al. Swellable and thermally responsive hydrogel/shape memory polymer foam composites for sealing lung biopsy tracts. ACS Biomater Sci Eng. 2023;9(2):642‐650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang Y, He J, Guo B. Functional hydrogels as wound dressing to enhance wound healing. ACS Nano. 2021;15(8):12687‐12722. [DOI] [PubMed] [Google Scholar]
- 31. Kharaziha M, Baidya A, Annabi N. Rational design of immunomodulatory hydrogels for chronic wound healing. Adv Mater. 2021;33(39):2100176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ishihara K, Shi X, Fukazawa K, Yamaoka T, Yao G, Wu JY. Biomimetic‐engineered silicone hydrogel contact lens materials. ACS Appl Bio Mater. 2023;6(9):3600‐3616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chatterjee S, Upadhyay P, Mishra M, et al. Advances in chemistry and composition of soft materials for drug releasing contact lenses. RSC Adv. 2020;10(60):36751‐36777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fea AM, Durr GM, Marolo P, Malinverni L, Economou MA, Ahmed I. XEN® gel stent: a comprehensive review on its use as a treatment option for refractory glaucoma. Clin Ophthal. 2020;14:1805‐1832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Long JE, Lee MS, Blithe DL. Update on novel hormonal and nonhormonal male contraceptive development. J Clin Endocrinol Metabol. 2021;106(6):e2381‐e2392. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data are available in the main text.


