Abstract
Objective: To evaluate the efficacy of FOLFOX regimen combined with cetuximab in the treatment of advanced colon cancer. Methods: This retrospective study involved 60 patients with primary colon cancer who were treated in the PLA Navy Anqing Hospital from January 2022 to February 2023. According to their treatment regimen, the patients were divided into a treatment group that received FOLFOX4 combined with cetuximab (n=30), and a control group treated with cetuximab alone (n=30). The general data of the two groups were compared, and the short-term response rate was assessed by comparing the proportions of complete remission (CR), partial remission (PR), stable disease (SD) and progressive disease (PD) between the two groups. In addition, the progression free survival (PFS) and overall survival (OS) were compared between the two groups, along with the adverse reactions and changes in serum tumor marker (CEA and CA19-9) levels. Result: The observation group showed a significantly higher short-term effective rate (CR+PR) compared to the control group (56.67% vs. 23.33%). The PFS and OS of the observation group were markedly longer compared to the control group. In terms of adverse reactions, the incidence of neutropenia, thrombocytopenia, nausea, vomiting, and diarrhea was similar between the two groups; however, the incidence of rash in the observation group was higher. After the treatment, the serum CEA and CA19-9 levels decreased markedly in both groups, and the observation group demonstrated obviously lower levels than the control group (P<0.001). Similarly, the decreases in VEGF-A and VEGFR2 levels in the observation group were more significant than those in the control group (all P<0.001). Conclusion: Despite inducing rash, which is controllable, the combined therapy of FOLFOX and cetuximab significantly improves short-term efficacy, reduces the levels of CEA, CA19-9, VEGF-A and VEGFR2, and extends the PFS and OS of patients, which can be served as an effective treatment strategy for advanced colon cancer.
Keywords: FOLFOX regimen, cetuximab, advanced colorectal cancer, tumor markers
Introduction
Chemotherapy is an important treatment method for colon cancer (CCA), significantly improving patient survival rate [1]. The FOLFOX regimen, a combination of folinic acid, 5-fluorouracil and oxaliplatin, is a first-line chemotherapy regimen for colon cancer. FOLFOX regimen inhibits the growth of tumor cells by interfering with DNA synthesis and replication. Cetuximab, a monoclonal antibody, inhibits the proliferation and metastasis of tumor cells by specifically blocking EGFR signaling pathways. However, the effectiveness of chemotherapy in CCA has reached a plateau, making the introduction of Cetuximab a promising development in targeted therapy for CCA. As a monoclonal antibody targeting the EGFR, cetuximab specifically binds to and blocks the EGFR signaling pathway, thereby inhibiting tumor cell growth and proliferation. The combination of cetuximab with chemotherapy can significantly improve the prognosis of patients with KRAS wild-type CCA. The selection of appropriate targeted drugs and their optimal combination to enhance quality of life has become a major focus of ongoing research [2].
Recent clinical trials have increasingly recognized cetuximab as a first-line treatment for CCA [3]. However, there is currently no consensus on how to optimize Cetuximab as the most optimal treatment for CCA. Additionally, there is a lack of research exploring the relationship between Cetuximab and CA19-9 levels, as well as drug resistance and progression-free survival (PFS) in advanced CCA treated with Cetuximab combined with first-line chemotherapy. The high cost of cetuximab combined with chemotherapy can also impose a significant financial burden on patients, leading to the decline of treatment in some eligible patients. Therefore, early evaluation of the efficacy of cetuximab combined with chemotherapy is of great significance in improving clinical prediction and patient confidence in the treatment. The 2012 European Society for Medical Oncology guidelines continued to recommend the combination of Cetuximab and FOLFOX as the first-line drug for CCA [4].
Tumor markers, substances produced by tumor cells or the body in response to them, are crucial for monitoring the occurrence, development, and prognosis of tumors, as well as their response to treatment. In colorectal cancer, CEA and CA19-9 are significant indicators. CEA, an acidic glycoprotein, is widely expressed in tumor tissue [5]. CA19-9 is a common mucinous carbohydrate expressed in the gastrointestinal, pancreatic, and other digestive systems of fetuses, with minimal expression in adults. Changes in CEA and CA19-9 are closely related to the therapeutic efficacy in tumors, making them valuable for evaluating treatment outcomes. While CEA and CA19-9 are important markers for assessing the effectiveness of oxaliplatin treatment, their relationship with cetuximab combined chemotherapy, resitance, efficacy, and survival time has yet to be reported.
The combination of Cetuximab with oxaliplatin-containing regimens remains controversial, and there is a lack of clinical research on the combination of Cetuximab and FOLFOX in the Chinese population, highlighting the need for further investigation. To address this gap, this study innovatively explored the differences in efficacy and safety between combined treatment of cetuximab and FOLFOX and cetuximab alone in Chinese CCA patients. It also assessed the levels of CEA and CA19-9 in first-line chemotherapy with Cetuximab and FOLFOX4, analyzing their correlation with chemotherapy sensitivity and prognosis. These findings provide a new theoretical basis for combining cetuximab and FOLFOX in treating KRAS wild-type colon cancer, offering valuable insights for personalized clinical treatment strategies.
Materials and methods
Research subject
This study retrospectively selected 60 patients with primary colon cancer treated at The PLA Navy Anqing Hospital from January 2022 to February 2023 after obtaining the ethical approval from the hospital ethics committee.
Inclusion criteria: (1) Aged 18 years and above; (2) Patients with metastatic colorectal cancer confirmed histopathologically for the first time [6]; (3) Expression of wild-type KRAS in tumor tissue; (4) No prior chemotherapy, or at least 6 months since the last use of adjuvant chemotherapy; (5) At least one measurable metastatic lesion confirmed by CT or MRI; (6) Normal blood routine, liver and kidney function; (7) Expected survival of more than 12 weeks.
Exclusion criteria: (1) Patients with severe heart disease, stroke, active infections, etc.; (2) Pregnant or lactating women; (3) Known hypersensitivity to cetuximab or any of the pharmaceutical ingredients in the FOLFOX regimen.
Research method
According to different treatment protocols, the patients received Cetuximab in combination with FOLFOX4 regimen were classified into the observation group (n=30), and those treated with cetuximab alone were classified into the control group (n=30).
(1) Control group (CG): Cetuximab (S20240025, Taizhou Mabtech Pharmaceutical Co., Ltd.) was initially administered at 400 mg/m2 via intravenous infusion for 2 hours, followed by a weekly dose of 250 mg/m2, with a maximum drip rate of 5 ml/min. To prevent allergic reactions, 30 minutes before treatment, patients received ranitidine (H20045516, Shanghai Hefeng Pharmaceutical Co., Ltd.) 50 mg, dexamethasone (H53021084, Longchuan Zhangfeng Pharmaceutical Factory) 5 mg, and diphenhydramine (H14022674, Shanxi Zhendong Anxin Biopharmaceutical Co., Ltd.) 50 mg via intramuscular injection. During the treatment, patients’ ECG, blood pressure, and blood oxygen levels were continuously monitored.
(2) Observation group (OG): Oxaliplatin (H20133094, Harbin Pharmaceutical Group Bioengineering Co., Ltd.) was intravenously administered at a dose of 85 mg/m2 on day 1 for 2 hours.
Calcium folinate (H20044159, Youcare Pharmaceutical Group Co., Ltd.) was intravenously administered at a dose of 200/m2 on days 1-2, over 2 hours.
Flumurizine (H21024236, Liaoning Xingao Pharmaceutical Co., Ltd.) was intravenously administered at a dose of 400/m2 on day 1 for 4 hours.
The regimen in both groups was repeated every 2 weeks, with efficacy evaluations every 8 weeks. Patients were withdrawn from the clinical study if their disease progressed.
(3) Data collection: Data collected from the study subjects included age, pathology, primary lesion morphology, KRAS gene status, adverse reactions (ARE) after treatment, treatment efficacy, and subsequent survival rates.
The therapeutic effect was evaluated according to RECIST 1.0 criteria. Complete remission (CR): Complete disappearance of the target lesion; Partial remission (PR): A reduction of more than 30% in the sum of the longest diameters (LD) of the target lesion compared to baseline; Progressive disease (PD): An increase of more than 20% in the LD of the target lesion compared to baseline, or the appearance of new lesions; Stable disease (SD): The lesion neither shrinks sufficiently to qualify as PR nor grows enough to qualify as PD. Treatment effective rate was defined as the sum of CR and PR. The control rate was defined as CR+PR+SD. The toxicity and side effects of the treatment were evaluated according to the NCI-CTC3.0 standard.
Serum levels of CEA (CSB-E04767h, Wuhan Huamei, China) and CA19-9 (CSB-E04773h, Wuhan Huamei, China) were measured using an enzyme linked immunosorbent assay (ELISA) according to the manufacturer’s instructions.
The serum levels of angiogenesis related indicators, VEGF (CSB-E11718h, Wuhan Huamei, China,) and VEGFR2 (CSB-E04763h, Wuhan Huamei, China), were also detected by ELISA.
Statistical analysis
SPSS 26.0 was used for data analysis. Measurement data were recorded as x̅±sd, and t-test was performed for inter-group comparison. Enumeration data were recorded as n (%), and χ2 test was performed for comparison between the two groups. Kaplan-Meier method was used to plot the overall survival curve over time. A p-value of less than 0.05 (P<0.05) was considered statistically significant.
Result
Comparison of baseline data between the two groups
As shown in Table 1, there were no statistical differences in sex, age, lesion morphology, or KRAS gene mutation status between the two groups (all P>0.05), indicating that the two groups were comparable.
Table 1.
Comparison of baseline data between the two groups
| Clinical features | Group | p | ||
|---|---|---|---|---|
|
|
|
|||
| Characteristic | Category | CG (n=30) | OG (n=30) | |
| Gender | Male | 21 | 22 | 0.658 |
| Female | 9 | 8 | ||
| Age (yrs) | 60.4±5.33 | 60.7±4.71 | 0.865 | |
| Lesion morphology | Protruded type | 11 | 12 | 0.756 |
| Ulcerative type | 12 | 11 | ||
| Infiltrative type | 7 | 7 | ||
| KRAS gene mutation | KRAS-wild | 21 | 22 | 0.867 |
| KRAS-mutant | 9 | 8 | ||
CG: control group; OG: observation group; KRAS: Kirsten rat sarcoma viral oncogene.
Comparison of short-term efficacy and survival between the two groups
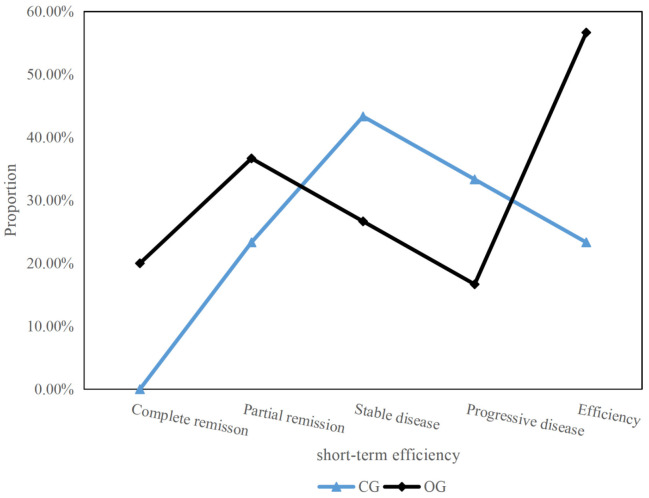
As shown in Figure 1, the OG showed significantly higher rates in CR and PR compared to the CG, resulting in significantly higher total response rate.
Figure 1.
Comparison of recent therapeutic effects. CG: control group; OG: observation group.
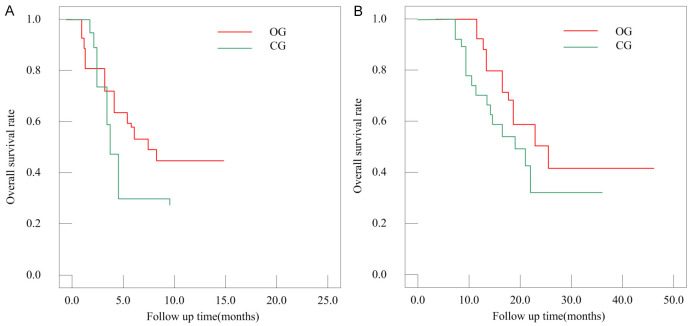
The progression-free survival (PFS) and overall survival (OS) were compared between the two groups (Table 2; Figure 2). The mean PFS in CG was 6.0±1.95 months, which was significantly shorter than 8.1±3.01 months in OG (P=0.03). The mean OS in CG was 17.8±5.15 months, also significantly shorter than 22.6±5.17 months in OG (P=0.01).
Table 2.
Comparison of survival data between the two groups
| Group | PFS (months) | OS (months) |
|---|---|---|
| CG | 6.0±1.95 | 17.8±5.15 |
| OG | 8.1±3.01 | 22.6±5.17 |
| t | 3.207 | 3.603 |
| p | 0.03 | 0.01 |
PFS: progression-free survival; OS: overall survival; CG: control group; OG: observation group.
Figure 2.
Overall survival curves. A: Progression free survival curve; B: Overall survival curve. CG: control group; OG: observation group.
Comparison of AREs between the two groups
As shown in Table 3, rash occurred in most patients of the OG (70%) but absent in CG (P<0.0001). However, no significant differences were observed in other ARES, such as neutropenia (P=0.684), thrombocytopenia (P=0.568), nausea and vomiting (P=1.000), or diarrhea (P=0.647).
Table 3.
Comparative AREs between the two groups [n (%)]
| Rash | Neutropenia | Thrombocytopenia | Nausea and vomiting | Diarrhea | |
|---|---|---|---|---|---|
| CG | 0 | 18 (60%) | 8 (26.67%) | 7 (23.33%) | 6 (20%) |
| OG | 21 (70%) | 20 (66.6%) | 7 (23.33%) | 7 (23.33%) | 7 (23.33%) |
| x2 | - | 0.287 | 0.089 | - | 0.098 |
| p | 0.000 | 0.684 | 0.568 | 1.000 | 0.647 |
CG: control group; OG: observation group; ARE: adverse reactions.
Changes in serum tumor markers CEA and CA19-9 levels
As shown in Table 4, there were no significant differences in pretreatment CEA (45.4±7.66 ng/mL vs. 43.2±8.43 ng/mL) and CA19-9 (72.1±12.13 U/mL vs. 75.4±15.93 U/mL) levels between the OG and CG (P=0.521, 0.416). However, after the treatment, the serum CEA level and CA19-9 level in OG were significantly lower than those in the CG (CEA: 9.3±1.62 ng/mL vs. 24.5±3.58 ng/Ml; CA19-9: 31.4±5.69 U/Ml vs. 50.1±12.25 U/Ml) (all P<0.0001).
Table 4.
Comparison of serum levels of CEA and CA19-9 between the two groups [n (%)]
| Group | n | CEA | CA19-9 | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | ||
| OG | 30 | 45.4±7.66 | 24.5±3.58 | 72.1±12.13 | 50.1±12.25 |
| CG | 30 | 43.2±8.43 | 9.3±1.62 | 75.4±15.93 | 31.4±5.69 |
| t | 0.884 | 4.245 | 0.821 | 6.428 | |
| P | 0.521 | <0.001 | 0.416 | <0.001 | |
CG: control group; OG: observation group; CEA: carcinoembryonic antigen.
Comparison of angiogenesis indicators between the two groups
As shown in Table 5, there were no significant differences in VEGF-A and VEGFR2 levels between the two groups before treatment. After treatment, the decrease in VEGF-A and VEGFR2 levels in the OG was more significant than those in OG (both P<0.0001).
Table 5.
Comparison of serum levels of VEGF and VEGFR2 between the two groups (x̅±sd)
| Group | n | VEGF/(pg·mL-1) | VEGFR2/(μmol·mL-1) | ||
|---|---|---|---|---|---|
|
|
|
||||
| Before treatment | After treatment | Before treatment | After treatment | ||
| OG | 30 | 814.31±71.66 | 532.77±58.57 | 480.77±51.33 | 301.77±35.63 |
| CG | 30 | 805.33±72.45 | 635.33±60.18 | 472.11±43.25 | 340.35±41.85 |
| t | 0.483 | 6.689 | 0.707 | 3.845 | |
| P | 0.631 | <0.001 | 0.483 | <0.001 | |
CG: control group; OG: observation group; VEGF: vascular endothelial cell growth factor; VEGFR2: vascular endothelial growth factor receptor 2.
Discussion
According to literature reports, the mutation rate (MRA) of KRAS gene in colorectal cancer ranges from 35% to 45% [7]. Studies have shown that the expression level of KRAS in colorectal cancer is closely related to the therapeutic effect of Cetuximab [8]. Research has found that patients with KRAS wild-type have significantly longer survival and PFS compared with patient with KRAS mutations [9,10]. This study found that the overall MRA of the KRAS gene is 32.4%, aligning with the mutation characteristics of the KRAS gene in Chinese population. At present, there is still significant controversy regarding the relationship between KRAS mutations and the clinical pathological characteristics of colorectal cancer. Wang et al. conducted a KRAS profiling analysis on 454 Chinese colorectal cancer patients and found that the frequency of KRAS gene mutations in patients over 60 years markedly exceeded that in patients younger than 60 years [11]. Wang et al. proposed that the KRAS gene mutation frequency in CCA patients with lung metastasis (LME) was higher than those without LME [12]. Research shows that the KRAS gene has a higher mutation frequency in women [13].
Numerous clinical trials have confirmed that Cetuximab+FOLFOX4 can be used as a first-line treatment regimen for KRAS wild-type CCA. OPUS trials have shown that the combination of Cetuximab and FOLFOX4 can significantly enhance efficacy and improve PFS. The combination of these two drugs extended the overall survival period of patients by 4 months compared to their single use [13]. Studies have found that Cetuximab+FOLFIRI combined with FOLFOX can significantly improve the remission rate of tumors. The combination of Cetuximab and FOLFOX chemotherapy has shown potential in shrinking tumors, making previously inoperable refractory colorectal cancer amenable to surgical intervention FOLFOX [14]. Ma et al. also found that the efficacy of Cetuximab+FOLFOX was significantly better than that of bevacizumab+FOLFOX. But the results of COIN and NORDIC VII were both negative, indicating that the combination of Cetuximab and FOLFOX is not more effective than using FOLFOX alone [15]. However, both clinical trials mentioned above have design flaws, and therefore their conclusions have been questioned by many scholars. There is currently no consensus on whether Cetuximab combined with FOLFOX4 can be used as a first-line treatment for KRAS wild-type CCA.
Early clinical exploration has confirmed that the combination of cetuximab and FOLFOX4 regimen can significantly improve the survival rate of KRAS wild-type cholangiocarcinoma (CCA) patients, laying a solid foundation for this combination therapy as a first-line treatment for KRAS wild-type CCA [16]. This article focuses on the combined effect of cetuximab and another chemotherapy combination FOLFOX3. Research data showed that in the observation group, patients treated with this combination regimen had a progression free survival (PFS) of 8.1±3.01 months and an overall survival (OS) of 22.6±5.17 months. Both key survival indicators showed significant advantages over the control group. According to authoritative literature at home and abroad, the efficacy of cetuximab as a first-line treatment in relevant tumor populations fluctuates between 44% and 77%, and the reported progression free survival generally ranges from 8 to 12 months [17]. The PFS data in this study are consistent with this range, further validating the clinical efficacy of the combination of cetuximab and FOLFOX3 regimen. It is worth noting that the majority of patients in this study exhibited high treatment compliance and were able to strictly adhere to the trial protocol, which is one of the key factors for successful treatment.
In this study, the patient’s side effects mainly included rash, hematotoxicity, nausea, vomiting, and diarrhea, with most of the side effects being mild and manageable. The outcomes demonstrated that the incidence of rash in the combination therapy group was 75.0%, aligning with most current literature reporting an overall prevalence of rash around 80% [18]. There was no significant difference in the incidence of other AREs. Additionally, no significant pulmonary toxicity reactions were found in this study. This may be because that all patients underwent strict anti-allergic preventive treatment before receiving Cetemovir.
Carcinoembryonic antigen (CEA) is a soluble acidic glycoprotein with six antigenic determinants [19]. It is present on tumor cells originating from endodermal tissues and can be detected in various bodily fluids such as blood and cerebrospinal fluid. CEA levels are generally low in healthy individuals but can be elevated in cases of benign liver diseases and in those with a history of smoking and drinking. CEA is a widely used tumor marker, particularly in colorectal cancer, where its positive rate ranges from 40% to 70%. It serves as a crucial indicator for prognosis, therapeutic response, and recurrence [19]. However, CEA testing can produce false positives and negatives, and its specificity is limited. Studies have shown that CEA levels correlate with colorectal cancer staging, with higher levels indicating advanced stages (e.g., 37% of patients with CEA>20 ng/ml are in stage D) [20]. Additionally, patients with high preoperative CEA levels have a significantly higher chance of postoperative recurrence [21]. CA19-9 is a glycosylated substance associated with the Lewis blood group. It is expressed in the gastrointestinal tract and pancreas but minimally expressed in healthy adults [22]. CA19-9 levels are particularly high in pancreatic cancer (71-93%), making it a valuable marker in this context. Elevated CA19-9 levels are linked to poor prognosis and higher recurrence rates in patients with cholangiocarcinoma (CCA) [23]. Although the sensitivity of CA19-9 for early diagnosis of CCA is low, combining CA19-9 with other markers like CEA improves diagnostic accuracy [24]. Our research demonstrated that the combination of CEA and CA19-9 provides high sensitivity (85.86%) and specificity (96.78%) in diagnosing colorectal cancer. In clinical trials, the FOLFOX regimen combined with cetuximab significantly reduced CEA and CA19-9 levels post-treatment, indicating its effectiveness.
VEGF-A is a key angiogenic factor that promotes the proliferation, migration and survival of vascular endothelial cells, thereby facilitating neovascularization. VEGFR2 is the primary receptor of VEGF-A, and their interaction activates several signaling pathways that promote angiogenesis and increase vascular permeability. Therefore, reducing the levels of VEGF-A and VEGFR2 can effectively inhibit angiogenesis, potentially slowing tumor growth and metastasis.
After treatment, VEGF-A and VEGFR2 levels decreased significantly more in the observation group than in the control group, suggesting that FOLFOX4 combined with cetuximab was more effective at inhibiting angiogenesis than cetuximab alone. In this study, the FOLFOX4 plus cetuximab regimen used in the observation group significantly reduced VEGF-A and VEGFR2 levels, likely due to the synergistic effect of both drugs. On the one hand, cetuximab can specifically bind to and block VEGFR2, thereby inhibiting VEGF-A signaling. On the other hand, FOLFOX4 as a chemotherapy regimen can directly kill or inhibit the growth of tumor cells, thus reducing VEGF-A production by tumor cells. This dual effect allowed the observation group to achieve more substantial reductions in VEGF-A and VEGFR2 levels.
Conclusion
The FOLFOX regimen combined with cetuximab has demonstrated significant efficacy in patients with advanced CCA, improving short-term outcomes, progression-free survival (PFS), and overall survival compared to cetuximab alone. While the incidence of rash was significantly higher in the treatment group, they were tolerable and manageable, and the incidence of other AREs was similar between the groups. Notably, this combination therapy effectively reduces CAE, CA19-9, VEGF-A and VEGFR2 levels, highlighting its potential advantage. In conclusion, the FOLFOX-cetuximab combination is a promising treatment strategy for advanced CCA, though further research is needed to validate its long-term efficacy and safety.
Disclosure of conflict of interest
None.
References
- 1.de Vries NL, van de Haar J, Veninga V, Chalabi M, Ijsselsteijn ME, van der Ploeg M, van den Bulk J, Ruano D, van den Berg JG, Haanen JB, Zeverijn LJ, Geurts BS, de Wit GF, Battaglia TW, Gelderblom H, Verheul HMW, Schumacher TN, Wessels LFA, Koning F, de Miranda NFCC, Voest EE. γδ T cells are effectors of immunotherapy in cancers with HLA class I defects. Nature. 2023;613:743–750. doi: 10.1038/s41586-022-05593-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng X, Ma S, Zhang Y, Lin W, Ji K, Wang C. In situ polymerization of fluorinated polyacrylate copolymer solid electrolytes for high-voltage lithium metal batteries at room temperature. Macromolecules. 2023;56:1077–1085. [Google Scholar]
- 3.Taieb J, Puig PL, Bedenne L. Cetuximab plus FOLFOX-4 for fully resected stage III colon carcinoma: scientific background and the ongoing PETACC-8 trial. Expert Rev Anticancer Ther. 2008;8:183–189. doi: 10.1586/14737140.8.2.183. [DOI] [PubMed] [Google Scholar]
- 4.Gallois C, Shi Q, Meyers JP, Iveson T, Alberts SR, de Gramont A, Sobrero AF, Haller DG, Oki E, Shields AF, Goldberg RM, Kerr R, Lonardi S, Yothers G, Kelly C, Boukovinas I, Labianca R, Sinicrope FA, Souglakos I, Yoshino T, Meyerhardt JA, Andre T, Papamichael D, Taieb J. Prognostic impact of early treatment and oxaliplatin discontinuation in patients with stage III colon cancer: an ACCENT/IDEA pooled analysis of 11 adjuvant trials. J. Clin. Oncol. 2023;41:803–815. doi: 10.1200/JCO.21.02726. [DOI] [PubMed] [Google Scholar]
- 5.Xu JF, Li XY, Long L, Liu R. Enhancement of the physical and mechanical properties of wood using a novel organo-montmorillonite/hyperbranched polyacrylate emulsion. Holzforschung. 2021;75:545–554. [Google Scholar]
- 6.Cervantes A, Adam R, Roselló S, Arnold D, Normanno N, Taïeb J, Seligmann J, De Baere T, Osterlund P, Yoshino T, Martinelli E ESMO Guidelines Committee. Electronic address: clinicalguidelines@esmo.org. Metastatic colorectal cancer: ESMO clinical practice guideline for diagnosis, treatment and follow-up. Ann Oncol. 2023;34:10–32. doi: 10.1016/j.annonc.2022.10.003. [DOI] [PubMed] [Google Scholar]
- 7.Peng S, Lu D, Zhang B, You R, Chen J, Xu H, Lu Y. Machine learning-assisted internal standard calibration label-free SERS strategy for colon cancer detection. Anal Bioanal Chem. 2023;415:1699–1707. doi: 10.1007/s00216-023-04566-1. [DOI] [PubMed] [Google Scholar]
- 8.Liang S, Li S, Yuan C, Zhang D, Chen J, Wu S. Polyacrylate backbone promotes photoinduced reversible solid-to-liquid transitions of azobenzene-containing polymers. Macromolecules. 2023;56:448–456. [Google Scholar]
- 9.Sun W, Liu T, Xia K, Zhou J, Liu X, Zhang X. Preparation of adsorbent based on polyacrylate latex solid waste and its application in the treatment of dye wastewater. ACS Omega. 2022;7:13243–13253. doi: 10.1021/acsomega.2c00690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hao Z, Li C, Han X, Liu K, Dai L, Si C. Multifunctional lignin-silver nanoparticles for accelerating polymerization and cross-linking of sodium polyacrylate. ACS Applied Polymer Materials. 2022;4:2140–2148. [Google Scholar]
- 11.Lu L, Li J, Wang F, Yan X, Qi D, Li X, Chen Y. Fluorosilicone modified polyacrylate/pigment hybrid latex: synthesis, properties, and application in binder-free pigment printing of polyester fabrics. Polym Adv Technol. 2022;33:904–914. [Google Scholar]
- 12.Al-Yaari M, Saleh TA. Mercury removal from water using a novel composite of polyacrylate-modified carbon. ACS Omega. 2022;7:14820–14831. doi: 10.1021/acsomega.2c00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuntyi O, Shepida M, Sozanskyi M, Sukhatskiy Y, Mazur A, Kytsya A, Bazylyak L. Sonoelectrochemical synthesis of silver nanoparticles in sodium polyacrylate solution. Biointerface Res Appl Chem. 2021;11:12202–12214. doi: 10.1155/2021/4465363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu S, Jin J. Radiotherapy guidelines for rectal cancer in China (2020 Edition) Precision Radiation Oncology. 2022;6:4–31. [Google Scholar]
- 15.Ma J, Yang QL, Ling Y. Rechallenge and maintenance therapy using cetuximab and chemotherapy administered to a patient with metastatic colorectal cancer. BMC Cancer. 2017;17:132. doi: 10.1186/s12885-017-3133-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Liu Z, Lin Q. Clinical effect and safety of targeted therapy combined with chemotherapy in the treatment of patients with advanced colon cancer. Pak J Med Sci. 2023;39:1074–1079. doi: 10.12669/pjms.39.4.7105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smithson M, Irwin RK, Williams G, McLeod MC, Choi EK, Ganguly A, Pepple A, Cho CS, Willey CD, Leopold J, Hardiman KM. Inhibition of DNA-PK may improve response to neoadjuvant chemoradiotherapy in rectal cancer. Neoplasia. 2022;25:53–61. doi: 10.1016/j.neo.2022.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Collard MK, Rullier E, Panis Y, Manceau G, Benoist S, Tuech JJ, Alves A, Laforest A, Mege D, Cazelles A, Beyer-Berjot L, Christou N, Cotte E, Lakkis Z, O’Connell L, Parc Y, Piessen G, Lefevre JH GRECCAR Group. Nonmetastatic ypt0 rectal cancer after neoadjuvant treatment and total mesorectal excision: lessons from a retrospective multicentric cohort of 383 patients. Surgery. 2022;171:1193–1199. doi: 10.1016/j.surg.2021.10.003. [DOI] [PubMed] [Google Scholar]
- 19.Mhaidly R, Mechta-Grigoriou F. Inflammatory fibroblasts make rectal cancer resistant to radiation therapy. Cancer Cell. 2022;40:122–124. doi: 10.1016/j.ccell.2022.01.005. [DOI] [PubMed] [Google Scholar]
- 20.Alagoa João A, Rocha R, Camarneiro R, Alves P, Carneiro C, Nunes V. Pull-through as an anastomotic salvage technique after taTME for low rectal cancer complicated by colon ischaemia - a video vignette. Colorectal Dis. 2022;24:342. doi: 10.1111/codi.16004. [DOI] [PubMed] [Google Scholar]
- 21.Akhmetzyanov FS, Egorov VI, Ruvinskiy DM, Lûtikov OV. The role of transanal drainage tube in low anterior resection for rectal cancer. Kazanskiĭ Meditsinskiĭ Zhurnal. 2021;102:335–341. [Google Scholar]
- 22.Lohynska R, Jirkovska M, Novakova-Jiresova A, Mazana E, Vambersky K, Veselsky T, Kindlova A, Stankusova H, Malinova B. Radiotherapy dose limit for uterus fertility sparing in curative chemoradiotherapy for rectal cancer. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2021;165:99–101. doi: 10.5507/bp.2020.039. [DOI] [PubMed] [Google Scholar]
- 23.Soda H, Maeda H, Hasegawa J, Takahashi T, Hazama S, Fukunaga M, Kono E, Kotaka M, Sakamoto J, Nagata N, Oba K, Mishima H. Multicenter phase II study of FOLFOX or biweekly XELOX and erbitux (cetuximab) as first-line therapy in patients with wild-type KRAS/BRAF metastatic colorectal cancer: the FLEET study. BMC Cancer. 2015;15:695. doi: 10.1186/s12885-015-1685-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sabuncuolu M Z, Zihni S, Szen S, Çelik G, Turan B, Acar S. Sponge single-port laparoscopy-assisted transanal total mesorectal excision for low rectal cancer: a technical report. Turkish Journal of Colorectal Disease. 2021;32:166–169. [Google Scholar]