Abstract
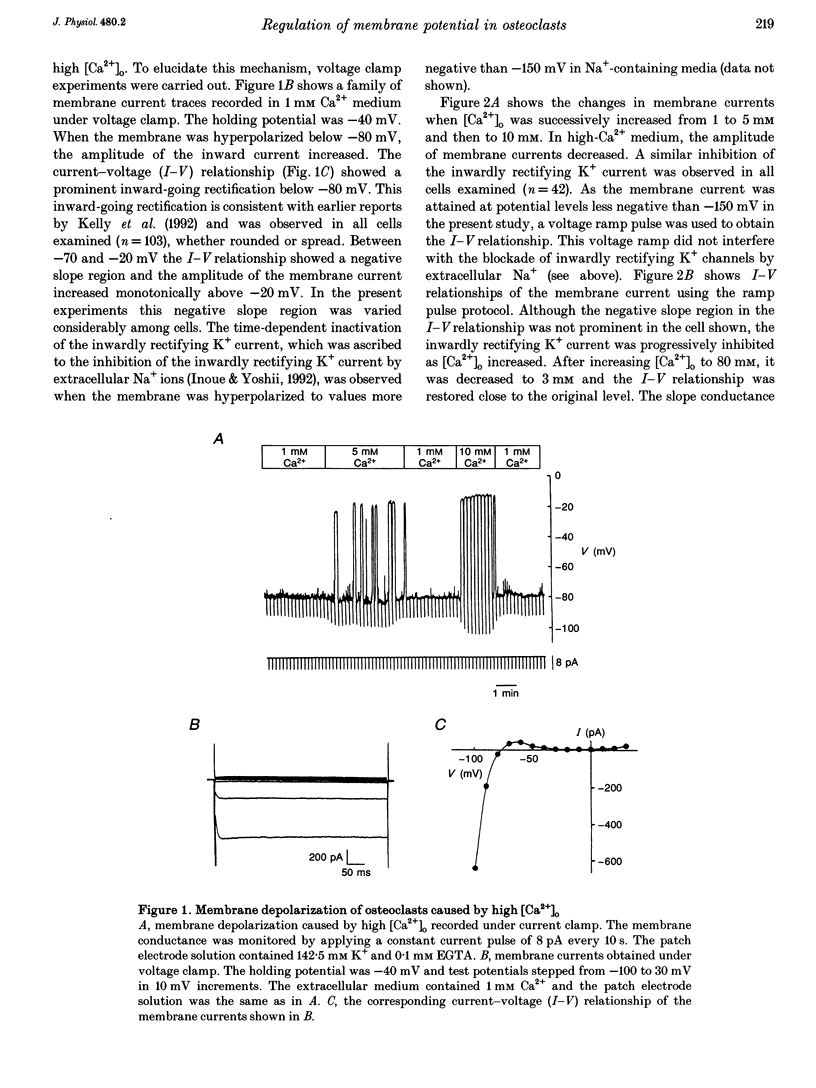
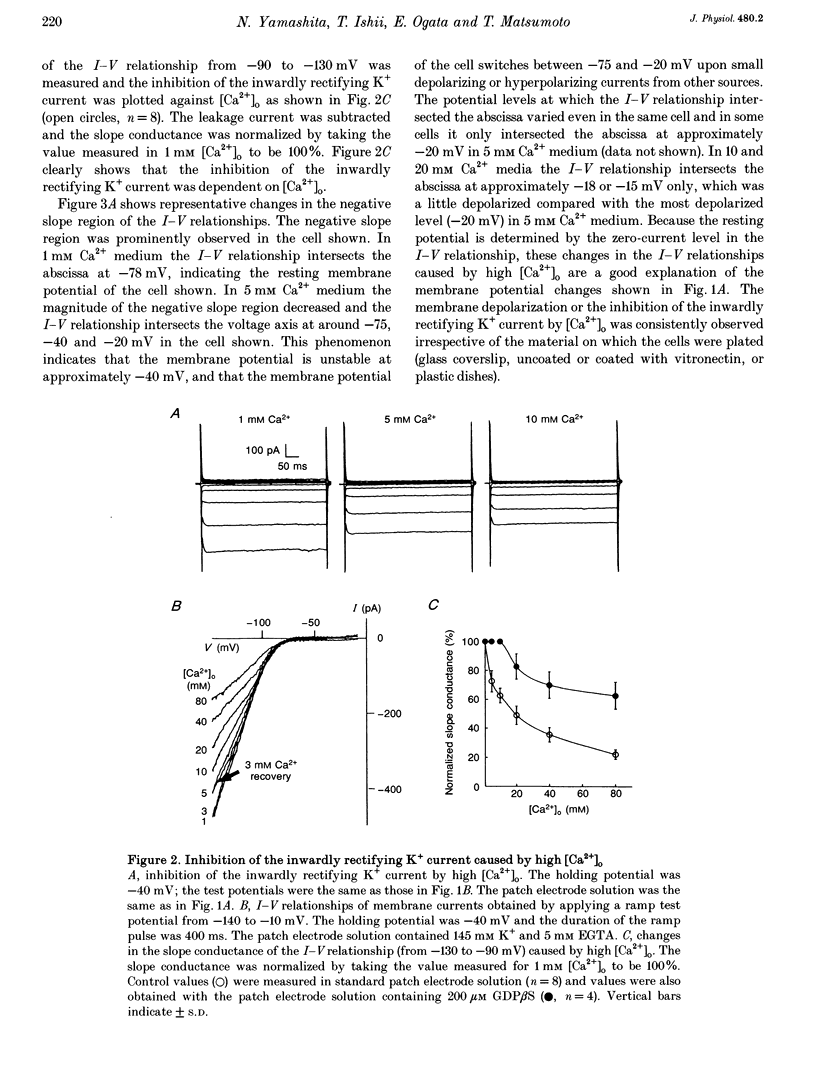
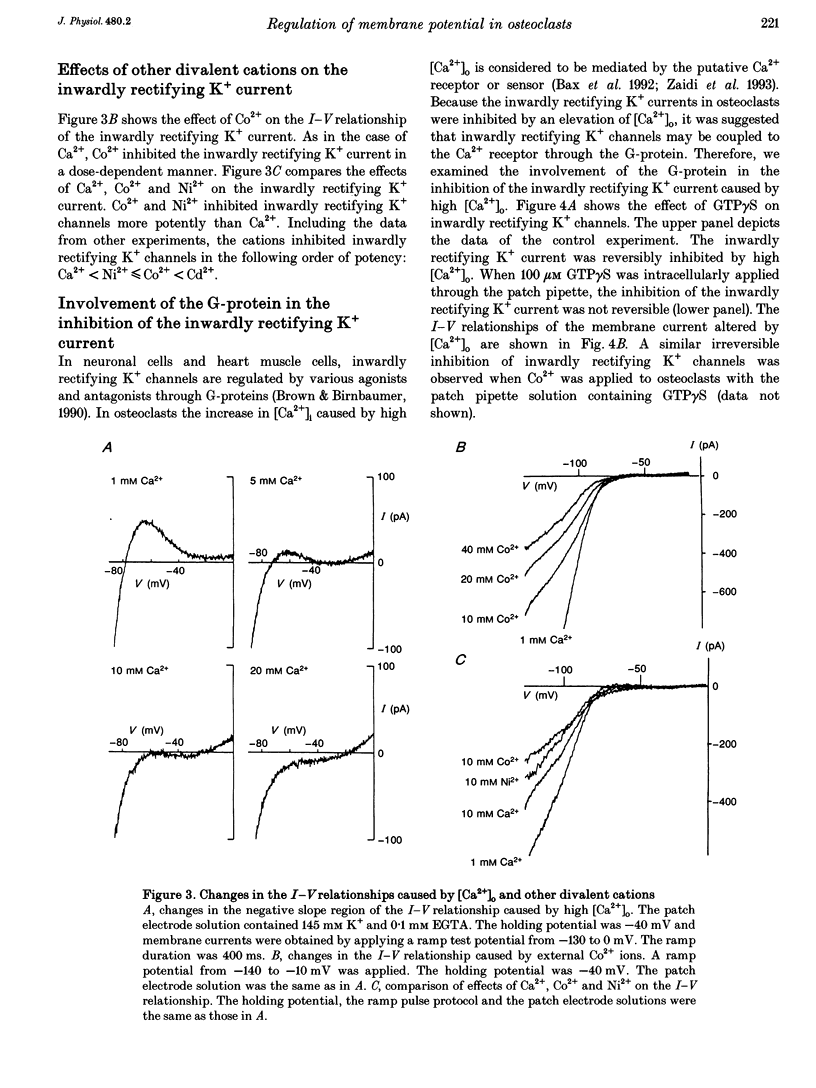
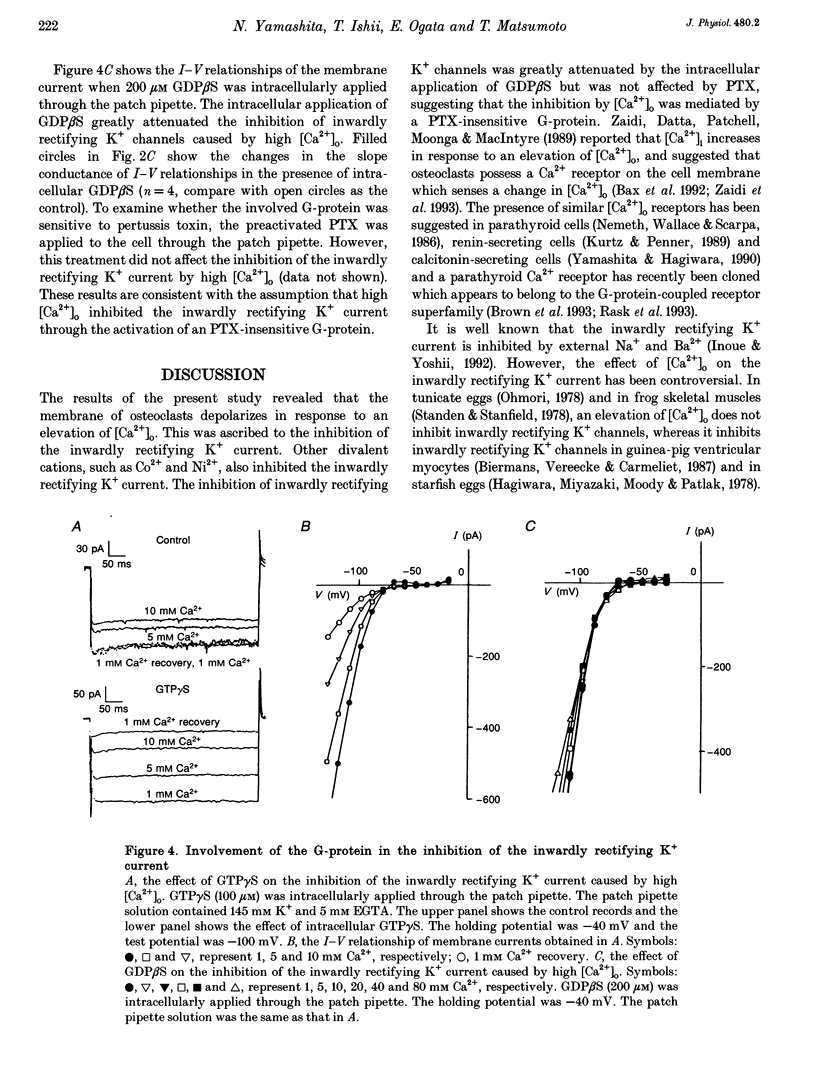
1. Regulation of membrane potential by extracellular Ca2+ concentration ([Ca2+]o) was examined in freshly isolated rabbit osteoclasts. 2. The resting membrane potential of osteoclasts was close to the K+ equilibrium potential in 1 mM Ca2+ medium. An elevation of [Ca2+]o caused membrane depolarization, accompanied by a decrease in the membrane conductance. 3. The inwardly rectifying K+ current observed under voltage clamp was dose-dependently inhibited by an elevation of [Ca2+]o, which explained the membrane depolarization caused by high [Ca2+]o. 4. Other divalent cations also inhibited the inwardly rectifying K+ current with the following order of potency: Ca2+ < Ni2+ < or = Co2+ < Cd2+. 5. In the presence of intracellular GTP gamma S the inwardly rectifying K+ current was irreversibly inhibited by [Ca2+]o, whereas the inhibition of the inwardly rectifying K+ current was greatly attenuated by intracellular application of GDP beta S. 6. Pertussis toxin (PTX) treatment did not abolish the inhibition of the inwardly rectifying K+ current caused by [Ca2+]o. 7. These results suggest that inwardly rectifying K+ channels in osteoclasts were regulated by a PTX-insensitive G-protein, which was coupled to the putative Ca2+ receptor or sensor on the cell membrane.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akisaka T., Gay C. V. Ultracytochemical evidence for a proton-pump adenosine triphosphatase in chick osteoclasts. Cell Tissue Res. 1986;245(3):507–512. doi: 10.1007/BF00218550. [DOI] [PubMed] [Google Scholar]
- Akisaka T., Yamamoto T., Gay C. V. Ultracytochemical investigation of calcium-activated adenosine triphosphatase (Ca++-ATPase) in chick tibia. J Bone Miner Res. 1988 Feb;3(1):19–25. doi: 10.1002/jbmr.5650030105. [DOI] [PubMed] [Google Scholar]
- Anderson R. E., Woodbury D. M., Jee W. S. Humoral and ionic regulation of osteoclast acidity. Calcif Tissue Int. 1986 Oct;39(4):252–258. doi: 10.1007/BF02555214. [DOI] [PubMed] [Google Scholar]
- Arkett S. A., Dixon S. J., Sims S. M. Substrate influences rat osteoclast morphology and expression of potassium conductances. J Physiol. 1992 Dec;458:633–653. doi: 10.1113/jphysiol.1992.sp019438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Neff L., Louvard D., Courtoy P. J. Cell-mediated extracellular acidification and bone resorption: evidence for a low pH in resorbing lacunae and localization of a 100-kD lysosomal membrane protein at the osteoclast ruffled border. J Cell Biol. 1985 Dec;101(6):2210–2222. doi: 10.1083/jcb.101.6.2210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron R., Neff L., Roy C., Boisvert A., Caplan M. Evidence for a high and specific concentration of (Na+,K+)ATPase in the plasma membrane of the osteoclast. Cell. 1986 Jul 18;46(2):311–320. doi: 10.1016/0092-8674(86)90748-8. [DOI] [PubMed] [Google Scholar]
- Bax C. M., Shankar V. S., Moonga B. S., Huang C. L., Zaidi M. Is the osteoclast calcium "receptor" a receptor-operated calcium channel? Biochem Biophys Res Commun. 1992 Mar 16;183(2):619–625. doi: 10.1016/0006-291x(92)90527-r. [DOI] [PubMed] [Google Scholar]
- Biermans G., Vereecke J., Carmeliet E. The mechanism of the inactivation of the inward-rectifying K current during hyperpolarizing steps in guinea-pig ventricular myocytes. Pflugers Arch. 1987 Dec;410(6):604–613. doi: 10.1007/BF00581320. [DOI] [PubMed] [Google Scholar]
- Brown A. M., Birnbaumer L. Ionic channels and their regulation by G protein subunits. Annu Rev Physiol. 1990;52:197–213. doi: 10.1146/annurev.ph.52.030190.001213. [DOI] [PubMed] [Google Scholar]
- Hagiwara S., Miyazaki S., Moody W., Patlak J. Blocking effects of barium and hydrogen ions on the potassium current during anomalous rectification in the starfish egg. J Physiol. 1978 Jun;279:167–185. doi: 10.1113/jphysiol.1978.sp012338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill O. P., Marty A., Neher E., Sakmann B., Sigworth F. J. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981 Aug;391(2):85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- Hunter S. J., Schraer H., Gay C. V. Characterization of isolated and cultured chick osteoclasts: the effects of acetazolamide, calcitonin, and parathyroid hormone on acid production. J Bone Miner Res. 1988 Jun;3(3):297–303. doi: 10.1002/jbmr.5650030308. [DOI] [PubMed] [Google Scholar]
- Inoue M., Yoshii M. Modulation of ion channels by somatostatin and acetylcholine. Prog Neurobiol. 1992;38(2):203–230. doi: 10.1016/0301-0082(92)90040-l. [DOI] [PubMed] [Google Scholar]
- Kelly M. E., Dixon S. J., Sims S. M. Inwardly rectifying potassium current in rabbit osteoclasts: a whole-cell and single-channel study. J Membr Biol. 1992 Mar;126(2):171–181. doi: 10.1007/BF00231915. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Penner R. Angiotensin II induces oscillations of intracellular calcium and blocks anomalous inward rectifying potassium current in mouse renal juxtaglomerular cells. Proc Natl Acad Sci U S A. 1989 May;86(9):3423–3427. doi: 10.1073/pnas.86.9.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyauchi A., Hruska K. A., Greenfield E. M., Duncan R., Alvarez J., Barattolo R., Colucci S., Zambonin-Zallone A., Teitelbaum S. L., Teti A. Osteoclast cytosolic calcium, regulated by voltage-gated calcium channels and extracellular calcium, controls podosome assembly and bone resorption. J Cell Biol. 1990 Dec;111(6 Pt 1):2543–2552. doi: 10.1083/jcb.111.6.2543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemeth E. F., Wallace J., Scarpa A. Stimulus-secretion coupling in bovine parathyroid cells. Dissociation between secretion and net changes in cytosolic Ca2+. J Biol Chem. 1986 Feb 25;261(6):2668–2674. [PubMed] [Google Scholar]
- Ohmori H. Inactivation kinetics and steady-state current noise in the anomalous rectifier of tunicate egg cell membranes. J Physiol. 1978 Aug;281:77–99. doi: 10.1113/jphysiol.1978.sp012410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravesloot J. H., Ypey D. L., Vrijheid-Lammers T., Nijweide P. J. Voltage-activated K+ conductances in freshly isolated embryonic chicken osteoclasts. Proc Natl Acad Sci U S A. 1989 Sep;86(17):6821–6825. doi: 10.1073/pnas.86.17.6821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silver I. A., Murrills R. J., Etherington D. J. Microelectrode studies on the acid microenvironment beneath adherent macrophages and osteoclasts. Exp Cell Res. 1988 Apr;175(2):266–276. doi: 10.1016/0014-4827(88)90191-7. [DOI] [PubMed] [Google Scholar]
- Sims S. M., Kelly M. E., Dixon S. J. K+ and Cl- currents in freshly isolated rat osteoclasts. Pflugers Arch. 1991 Oct;419(3-4):358–370. doi: 10.1007/BF00371118. [DOI] [PubMed] [Google Scholar]
- Standen N. B., Stanfield P. R. A potential- and time-dependent blockade of inward rectification in frog skeletal muscle fibres by barium and strontium ions. J Physiol. 1978 Jul;280:169–191. doi: 10.1113/jphysiol.1978.sp012379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezuka K., Sato T., Kamioka H., Nijweide P. J., Tanaka K., Matsuo T., Ohta M., Kurihara N., Hakeda Y., Kumegawa M. Identification of osteopontin in isolated rabbit osteoclasts. Biochem Biophys Res Commun. 1992 Jul 31;186(2):911–917. doi: 10.1016/0006-291x(92)90832-6. [DOI] [PubMed] [Google Scholar]
- Tuukkanen J., Vänänen H. K. Omeprazole, a specific inhibitor of H+-K+-ATPase, inhibits bone resorption in vitro. Calcif Tissue Int. 1986 Feb;38(2):123–125. doi: 10.1007/BF02556841. [DOI] [PubMed] [Google Scholar]
- Yamashita N., Hagiwara S. Membrane depolarization and intracellular Ca2+ increase caused by high external Ca2+ in a rat calcitonin-secreting cell line. J Physiol. 1990 Dec;431:243–267. doi: 10.1113/jphysiol.1990.sp018329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita N., Shibuya N., Ogata E. Requirement of GTP on somatostatin-induced K+ current in human pituitary tumor cells. Proc Natl Acad Sci U S A. 1988 Jul;85(13):4924–4928. doi: 10.1073/pnas.85.13.4924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaidi M., Alam A. S., Huang C. L., Pazianas M., Bax C. M., Bax B. E., Moonga B. S., Bevis P. J., Shankar V. S. Extracellular Ca2+ sensing by the osteoclast. Cell Calcium. 1993 Apr;14(4):271–277. doi: 10.1016/0143-4160(93)90048-b. [DOI] [PubMed] [Google Scholar]
- Zaidi M., Datta H. K., Patchell A., Moonga B., MacIntyre I. 'Calcium-activated' intracellular calcium elevation: a novel mechanism of osteoclast regulation. Biochem Biophys Res Commun. 1989 Sep 29;163(3):1461–1465. doi: 10.1016/0006-291x(89)91143-1. [DOI] [PubMed] [Google Scholar]


