Abstract
INTRODUCTION
Upper limb impairment is a common consequence of stroke, significantly affecting the quality of life and independence of survivors. This scoping review assesses the emerging field of muscle synergy analysis in enhancing upper limb rehabilitation, focusing on the comparison of various methodologies and their outcomes. It aims to standardize these approaches to improve the effectiveness of rehabilitation interventions and drive future research in the domain.
EVIDENCE ACQUISITION
Studies included in this scoping review focused on the analysis of muscle synergies during longitudinal rehabilitation of stroke survivors’ upper limbs. A systematic literature search was conducted using PubMed, Scopus, and Web of Science databases, until September 2023, and was guided by the PRISMA for scoping review framework.
EVIDENCE SYNTHESIS
Fourteen studies involving a total of 247 stroke patients were reviewed, featuring varied patient populations and rehabilitative interventions. Protocols differed among studies, with some utilizing robotic assistance and others relying on traditional therapy methods. Muscle synergy extraction was predominantly conducted using Non-Negative Matrix Factorization from electromyography data, focusing on key upper limb muscles essential for shoulder, elbow, and wrist rehabilitation. A notable observation across the studies was the heterogeneity in findings, particularly in the changes observed in the number, weightings, and temporal coefficients of muscle synergies. The studies indicated varied and complex relationships between muscle synergy variations and clinical outcomes. This diversity underscored the complexity involved in interpreting muscle coordination in the stroke population. The variability in results was also influenced by differing methodologies in muscle synergy analysis, highlighting a need for more standardized approaches to improve future research comparability and consistency.
CONCLUSIONS
The synthesis of evidence presented in this scoping review highlights the promising role of muscle synergy analysis as an indicator of motor control recovery in stroke rehabilitation. By offering a comprehensive overview of the current state of research and advocating for harmonized methodological practices in future longitudinal studies, this scoping review aspires to advance the field of upper limb rehabilitation, ensuring that post-stroke interventions are both scientifically grounded and optimally beneficial for patients.
Key words: Muscles, Upper extremity, Stroke rehabilitation, Electromyography, Longitudinal studies
Introduction
Stroke is a major global health burden, ranking as the second-leading cause of mortality and the third-leading cause of disability-adjusted life years (DALYs) worldwide.1 A common sequelae of stroke is upper limb paresis, which affects up to 80% of stroke survivors and impairs their ability to perform activities of daily living.2 The measurement of upper limb function is essential for assessing rehabilitation therapies’ effectiveness and enhancing therapeutic practice.
Several common assessments are used to evaluate upper limb function in a stroke patient, such as Motor Activity Log (MAL), the Wolf Motor Function Test (WMFT), Fugl-Meyer assessment (FMA), Action Research Arm Test (ARAT) and other clinical measures.3 These tools are designed to gauge motor function and recovery.4 However, the data obtained from these measures can be influenced by various factors such as the examiner’s experience and can be sensitive to subjective interpretation.5 Moreover, these methods mainly describe residual function without focusing on the neural representation of upper limb coordination.6 The complexity of the upper limb movements during normal daily activities and the different phenotypes of upper limb post-stroke are primarily described by kinematics and muscle activations.7 In fact, muscle activation patterns are often impaired after stroke, which can limit both movement coordination and functional recovery. Therefore, more dynamic and objective measures are needed to quantify these movement-related characteristics and analyze the neural basis of abnormal movement in stroke.8
Historically, abnormal synergies are clinically described as stereotyped movements due to the loss of independent joint control often defined as the flexion synergy and the extension synergy.9 Indeed, abnormal muscle synergies tend to express after the first three-six months post stroke particularly in moderate severe impaired stroke patients and can limit both movement coordination and functional recovery.10 In fact, synergies are closely linked with motor recovery after stroke.11 The study of muscles synergies has become important for improving the understanding of motor impairment and recovery after stroke, and for developing new rehabilitation therapies that can improve patient outcomes.12 This approach holds promise for various fields, including sports science, prosthetics, and neurorehabilitation.
Neural basis of upper limb muscle synergies
Several models and analysis techniques have been employed to describe the neural representation of upper limb movement, with the goal of capturing its hierarchical and modular organization. These models include somatotopy, cortical connectivity, and muscle synergies.10 In particular, the analysis of muscle synergies, extracted from electromyography (EMG) data derived from multiple muscles, has proved its potential to provide a quantitative representation of the neural mechanisms that underlie human movement in many scenarios including patients’ evaluation.
Muscle synergies are coordinated muscle activation patterns, governed by dynamic neural commands indicating a functional neural linkage.13 This has been supported by numerous animal studies, which propose a linear combination of synergies as the basic blocks underlying movement.13, 14 These synergies are believed to mirror the connections among motoneurons in the spinal cord, triggered by central commands and sensory feedback.10
The muscle synergy theory aligns with the dynamic-systems model, viewing movement as an interplay among various dynamic systems – namely nervous system, and environment. In this context, muscle synergies function as modules that the central nervous system (CNS) can activate or modify to meet task demands and environmental conditions.15 Examining how muscle synergies encode task parameters can provide insights into the organization of human motor control,16 illuminating the integration and coordination of various motor command levels to yield adaptive, flexible movements. One recent study scrutinized spatial and temporal synergies during multi-joint upper limb movement, stressing the importance of characterizing motor coordination at both temporal and spatial levels to comprehend the neurophysiological mechanisms governing upper limb movement.17 Furthermore, d’Avella et al. demonstrated that specific time-varying (spatiotemporal) muscle synergies underpin the execution of varied motor tasks such as defensive kicking in frogs, and could accurately reconstruct the muscle activation patterns required for fast-reaching movements in humans.13, 18
Despite ongoing debate regarding the neural mechanisms underlying muscle synergies, they provide valuable insights into the neural coordination of muscles.19 Evidence supporting a neural origin of muscle synergies comes from vertebrate studies,19 developmental observations of synergies shared across behaviors and species,20-22 and clinical findings of synergies preserved after stroke.23 In contrast, others propose that biomechanical constraints could explain synergies without requiring neural coupling.24-26 Using cadaver experiments and computational models, it has been demonstrated that constraints imposed by the limb biomechanics can produce apparent muscle couplings even if muscles are assumed to be independently controlled.27 As a synthesis, Ting and McKay propose that muscle synergies reflect a neural control strategy that incorporates biomechanical constraints.28 They argue synergies emerge from the interplay between neural control and the biomechanics of the body and environment. Alessandro et al. proposed a shift towards a more task-oriented analysis by evaluating whether muscle synergies can effectively generate the required task-level behaviors. This approach would provide stronger evidence to resolve the debate on whether synergies reflect the neural control strategies used by the CNS, demonstrating that synergies enable the nervous system to achieve task-level goals by coordinating muscles in a manner that accounts for the dynamics of the musculoskeletal system.29
Computationally identified muscle synergies
Computationally, muscle synergies are typically extracted from the processed EMG signals using several matrix decomposition techniques. The most common method is Non-Negative Matrix Factorization (NMF), which factors the EMG matrix into two non-negative matrices: the synergy matrix (W) and the activation coefficient matrix (C).30 The synergy matrix represents the relative weighting of each muscle within each synergy, while the activation coefficient matrix represents the time-varying activation of each synergy during the movement. Mathematically, the EMG matrix (m × t), where m is the number of muscles and t is the number of time points, is decomposed into the product of the synergy matrix (m × n) and the activation coefficient matrix (n × t), where n is the number of synergies.18 Each column of the synergy matrix represents a single synergy, with the values indicating the relative contribution of each muscle to that synergy. Each row of the activation coefficient matrix represents the time-varying activation of a specific synergy throughout the movement. Additionally, other algorithms including Principal Component Analysis (PCA),31 Independent Component Analysis (ICA)32 and Factor Analysis18 are also utilized. The number of synergies (n) is usually determined by iteratively increasing the number of synergies until the incremental increase in Variance Accounted For (VAF) or R2 becomes negligible. Both VAF and R-squared measure the percentage of the original EMG data that can be reconstructed using the extracted synergies.33
Muscle synergies and stroke
After a stroke, damage to key motor pathways, such as the corticospinal tract, can disrupt the normal control of movement and lead to the development of abnormal synergies. To date, some cross-sectional studies have provided some insights into the post-stroke muscle motor function based on muscle synergies.7, 8, 23, 34-37 Cheung et al. highlighted the stability of muscle synergies post-stroke, suggesting that despite cortical damage, the CNS retains some aspects of pre-stroke motor coordination.23, 35 This stability refers to the similarity of the basic structure of muscle synergies between affected and unaffected arm.23 However, while the structure of these synergies is conserved, the numbers of muscle coordination can be represented by three patterns such as preservation, merging and fractionation.35 These patterns were found to correlate with the level of motor impairment, as assessed by the Fugl-Meyer Scale, and time since the stroke onset.35 García-Cossio et al. found that new synergy patterns were generated in severe affected upper limb depending only in case of intact sensorimotor cortex.37 Building on these insights, McMorland et al. in 2015 emphasized that recovery hinges on two factors: the reorganization of impaired synergies and the formation of new ones through cortical reorganization.10 Conversely, Roh et al.,7, 36 Pan et al.8 and Li et al.34 reported no significant difference in number of synergies between stroke and control groups. However, they observed changes in the structure of muscle synergies, with these changes correlating with the severity of motor deficits in chronic and subacute stroke patients.
Muscle synergies and stroke rehabilitation
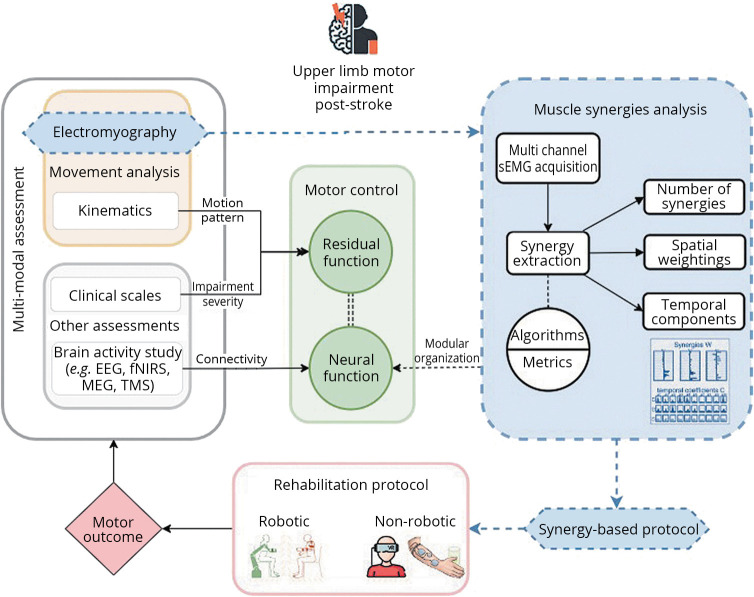
Stroke rehabilitation for upper limb impairment can involve a variety of approaches, including physical therapy, occupational therapy, and the use of assistive technologies, robots, brain computer interface (BCI), virtual reality (VR),38 and others. While the effectiveness of stroke rehabilitation can vary depending on several factors such as the severity of the stroke, the individual’s level of motivation and engagement in therapy, researchers have demonstrated that early and intensive rehabilitation can lead to better outcomes in terms of functional recovery and overall independence.39 In the last 20 years, there has been an increasing interest in the use of robotics and other technologies for upper limb rehabilitation following stroke.40, 41 These technologies can provide intensive and repetitive training, which is essential for promoting neural plasticity and functional recovery since the early phase of stroke.42 Robotic devices can also provide objective measurements of performance and progress and enable customized training programs tailored to the specific needs and abilities of individual patients.43 However, despite promising results, further research is needed to better understand the mechanisms underlying the effects of these technologies and to optimize their use in stroke rehabilitation.44 To maximize the benefits of these technologies, it is crucial to supplement them with comprehensive models that explain and interpret the performance of each body part and its function, all within the context of a unified theory of motor control.45 Figure 1 illustrates a framework for post-stroke upper limb rehabilitation, adopting a multi-modal, motor control-based approach that incorporates muscle synergy analysis to customize treatment protocols aimed at enhancing motor outcomes.
Figure 1.
—A framework for stroke rehabilitation: a multi-modal assessment strategy. sEMG: surface electromyography; EEG: electroencephalography; fNIRS: functional near infrared spectroscopy; TMS: transcranial magnetic stimulation; MEG: magnetoencephalography. This diagram outlines a framework for stroke rehabilitation, encompassing a multi-modal assessment strategy. Key components include movement analysis, via electromyography and kinematics, brain activity assessments such as EEG, fNIRS, TMS, MEG and clinical scale evaluations, all of which contribute to an understanding of neural and residual motor functions. Dotted lines highlight the role of muscle synergy analysis in fine-tuning the rehabilitation protocol, which is continuously refined to enhance motor outcomes, suggesting that the integration of synergy data can lead to adjustments for more targeted intervention.
In the recent Robot Assisted Training for the Upper Limb after Stroke (RATULS) Trial, the largest multicentered study in the field of robot-assisted upper limb training, the outcomes demonstrated the unequivocal benefits of neurorehabilitation for post-stroke motor recovery.46 However, robot-assisted training did not exhibit a marked superiority in augmenting upper limb functional outcomes compared to “enhanced upper limb therapy” measured with ARAT. While the ARAT provided valuable metrics for evaluation, it may not fully capture the intricate dynamics of motor neuroplasticity. Muscle synergy analysis offers a promising approach to assess motor function and elucidate the complex interplay of motor control mechanisms post-stroke. By examining coordinated muscle activation patterns, muscle synergies afford a deeper insight into the subtle yet critical differences in motor control restoration attributed to diverse rehabilitation modalities. Applied to studies similar to RATULS, muscle synergy analysis could provide valuable insights into the underlying neural mechanisms of motor recovery and help identify the most effective rehabilitation strategies.46
Furthermore, muscle synergies have the potential to guide the development of personalized therapeutic protocols in stroke rehabilitation. A review by Hong et al. suggests that models combined with robotic exoskeletons may enhance rehabilitation outcomes through synergy-based training47, with only three studies focused on upper limb treatment in stroke patients. A thorough literature review revealed that rehabilitation can lead to a structure and timing of muscle synergies during walking in stroke patients more similar to those observed in healthy individuals.48
Despite these promising results, it remains unclear how longitudinal rehabilitative treatments influence muscle synergies and how synergy-based treatments might guide personalized treatments, thereby improving rehabilitative approaches. The organization of muscle synergies into modular and fractionated patterns serves as both an indicator of motor impairment and a potential target for neurorehabilitation.35 These patterns can be manipulated to create new synergies, alter activation profiles, and modify internal structure, thereby improving motor function. This scoping review aims to investigate the impact of rehabilitative interventions on upper limb muscle synergies in stroke patients. The review will examine how muscle synergy analysis is employed with various rehabilitation approaches and integrated with clinical scales and instrumental assessments. By identifying gaps in the existing literature, this review will provide preliminary recommendations for designing effective interventional studies on muscle synergies in stroke populations.
Evidence acquisition
Study approach
This paper appraises or evaluates the current use of muscle synergies for the analyses or the assessment of post-stroke upper limb longitudinal interventions.
Inclusion criteria were: 1) muscle synergies analysis in longitudinal upper limb rehabilitation in stroke patients; 2) post-stroke upper limb function quantification; 3) protocols and set-ups based on muscle synergies; 4) interventions using conventional and innovative technologies. Articles analyzing subjects with spinal cord injury, multiple sclerosis, Parkinson’s disease, and other diseases that interfere with upper limb function were excluded. Single-session assessment studies were also excluded, as the focus was on longitudinal assessments. There was no exclusion based on study quality or methodology, given the limited number of studies in this field. In this scoping review, we categorized studies into robotic and non-robotic rehabilitation interventions based on muscle synergies in post-stroke upper limb rehabilitation. This distinction is crucial for understanding the unique impacts of each intervention type on muscle coordination. Robotic interventions, characterized by their specific, repetitive movements, offer a distinct technological influence on muscle synergy patterns, although they are not yet fully personalized to patient needs. This approach aims to enhance our understanding of muscle synergies application across diverse rehabilitation modalities.
Three independent authors systematically screened each record and report to determine eligibility based on the predefined inclusion/exclusion criteria and disagreements were resolved through discussion. Subsequent data collection from the selected reports was also performed, verifying accuracy through cross-checks and, when necessary, seeking confirmation from the original investigators. The measurement methods were studied in detail, and the parameters considered were identified and charted.
More specifically, this scoping review attempts to answer the main research question (RQ 0): “How have muscle synergies been used in clinical practice for assessment of upper limb interventions in post-stroke rehabilitation?”.
RQ 0 is further divided into the following research questions:
RQ (1) What is the impact of longitudinal interventions on the structure, number, and temporal evolution of muscle synergies?
RQ (2) How do the algorithms used, selection of muscles and stroke characteristics influence the extraction and interpretation of muscle synergies?
RQ (3) How do changes in muscle synergies correlate with clinical outcomes and other metrics of stroke recovery throughout treatment?
RQ (4) What differences were implemented between the rehabilitation protocols and synergy extraction acquisition sessions?
RQ (5) How do task-specific differences in muscle synergies inform the design of personalized rehabilitation protocols?
This scoping review employed the Joanna Briggs Institute (JBI) methodology,49 aiming to explore the breadth of literature on muscle synergy analysis in post-stroke upper limb rehabilitation. The scoping review was guided by the PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) framework for scoping reviews.50 The international guidelines established by PRISMA 2020 were followed.51 To ensure a comprehensive answer to the research questions, all the included articles were thoroughly reviewed, and pertinent data were systematically extracted. Data from the included studies were charted using a customized Microsoft Excel spreadsheet. The extracted data focused on study characteristics, types of interventions, clinical outcomes, muscle synergy analysis and suggestions for future research. A narrative synthesis approach was utilized to summarize the findings and present them in alignment with the review objectives.
Search strategy
With the above-mentioned aims, the following procedure was employed for the literature screening. A comprehensive literature search was obtained by screening PubMed, Scopus, and Web of Science, using a query based on the keywords: Muscle synerg* AND Rehabilitation AND stroke, “upper limb” OR “upper extremity”. The search queries conducted across various databases were as follows:
1) Web of Science: ALL=(“muscle synerg*”) AND ALL=(stroke) AND ALL=(rehabilitation) AND ALL=(“upper limb” OR “upper extremity”).
2) PubMed: (((muscle synerg*) AND stroke) AND rehabilitation) AND ((upper limb) OR (upper extremity)).
3) Scopus: TITLE-ABS-KEY (“muscle synerg*”) AND TITLE-ABS-KEY (stroke) AND TITLE-ABS-KEY (rehabilitation) AND TITLE-ABS-KEY (“upper limb” OR “upper extremity”).
The full text of the relevant articles was reviewed to determine which ones met the study criteria for further analysis. The search was limited to articles published in English up to September 2023. In addition, the references of the relevant articles were checked to identify any additional studies that could be included in this analysis. A total of 314 papers were identified. For a visual representation of the article selection process, refer to Figure 2.
Figure 2.
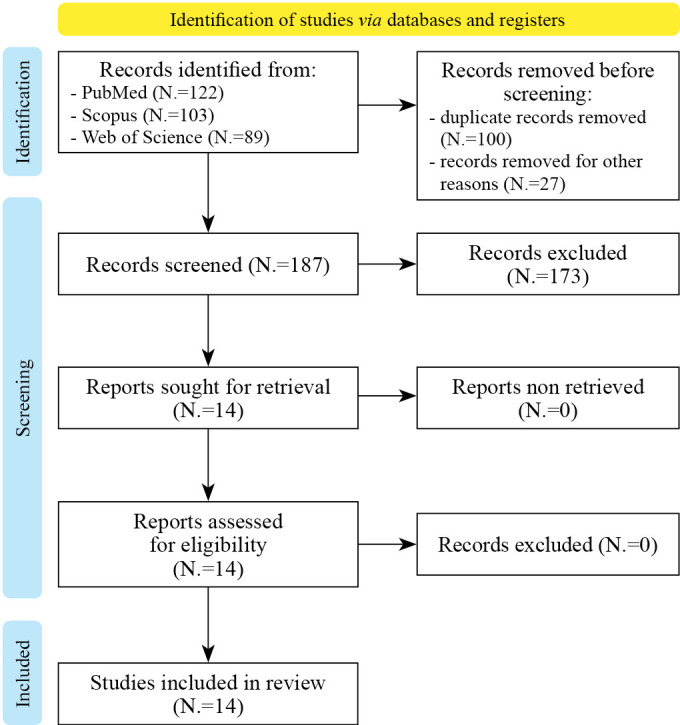
—The PRISMA flow chart for the proposed scoping review.
Evidence synthesis
In this scoping review, a total of 14 articles involving a total of 247 stroke patients were included. Ten studies employed an observational pre-post experimental design,52-59 Lencioni et al., Niu et al. and Zendehbad et al., which utilized a randomized controlled trial design.60-62 Two studies performed a secondary analysis of muscle synergy based on previously published works.63, 64 Particularly, Hesam Shariati et al. published their paper in two parts: the first part showed clinical evaluations and outcomes and the second part focused on muscle synergies analysis in the same population.63, 65 Seo et al. utilized extracted sEMG data from an existing randomized controlled trial by Mugler et al66 for muscle synergies analysis.64 Accordingly, clinical outcomes and kinematics data were sourced from the study conducted by Mugler et al. for the purpose of this review.66
The characteristics of the studies in this scoping review differ depending on the rehabilitative therapies used, the tasks employed, the outcome measurements, and the synergy-related analysis, as indicated in Supplementary Digital Material 1, Supplementary Table I, II. Due to these variations, the characteristics of the synergy-related outcomes varied across the studies. In this analysis, two groups of studies have been considered: those that used robotics devices and those that used other rehabilitation approaches.
Robotic studies
Enrolled patients and volunteers
In the six studies based on robotic intervention, we observed a diverse range of populations. The number of participants in these studies ranged from a small sample size of five chronic stroke patients to larger cohorts, up to 32 patients. Pierella et al. enrolled 6 subacute stroke patients and 6 healthy controls, for a total of 12 participants.52 Scotto di Luzio et al. studied 7 chronic stroke patients.53 Tropea et al. included 6 subacute stroke patients and 10 healthy controls, totaling 16 subjects.55 Belfatto et al. analyzed data from 5 chronic stroke patients, which is the smallest sample size among the robotic studies reviewed.54 Irastorza-Landa et al. recruited the largest number of stroke patients, with 18 chronic stroke patients divided into an experimental group of 10 and a control group of 8.56 Lencioni et al. studied 32 stroke patients, divided into a robotic group of 15 and a usual care group of 17, along with 10 healthy controls.60 In the Lencioni et al., Irastorza et al. and Scotto di Luzio et al., paretic and ipsilesional limbs were compared.53, 56, 60 All participants in Pierella et al.’s study had ischemic strokes and were right-side paretic.52 In contrast, Tropea et al.’s study included five ischemic and one hemorrhagic stroke patients, with the majority being left-side paretic.55 Two studies did not specify the type of stroke or affected side in their population.54, 56
Turning to the evaluation metrics, the FMA stands out as a prominent tool for assessing upper limb motor impairment.67 It is worth noting that the FMA-UL comprises 33 items and each item is scored as 0 (indicating absence of function), 1 (partial impairment), or 2 (no impairment), allowing for a cumulative score range from 0 to 66. The authors across studies employed different subsets of the FMA domains to calculate the final score. Pierella et al. presented a range of FMA-UL scores between 5 to 54, encompassing both mild, moderate and severe impairments.52 Scotto di Luzio et al. enrolled a narrower range of patients, with FMA-UL scores between 26 and 45, indicating a moderate to severe motor impairment.53 Another study reported FMA-UL scores between 8 and 36, which suggests that the patients had severe motor impairment.55 Belfatto et al. used FMA-UL (sections A-D), with scores ranging from 11 to 61, suggesting a broad spectrum of motor impairment levels.54 Irastorza et al. used the modified FMA-UL, scores ranged from 2 to 33, pointing to a severely impaired group.56 Finally, Lencioni et al. reported FMA-UL median scores of 45 in the robotic group and 21 in the usual care group.60
Types of intervention
Various robotic devices were employed across the studies to facilitate the rehabilitation process, each tailored to specific aspects of upper limb rehabilitation. The InMotion2 robot (Interactive Motion Technologies, Inc., Cambridge, MA, USA) was utilized in the study by Tropea et al. to enable patients to perform reaching movements in the eight cardinal directions on a horizontal plane, offering assistance as needed.55 The Braccio di Ferro system (Celin s.r.l., Italy), as used in the study by Lencioni et al., allowed participants to control the position of an end-effector in planar movements, focusing on reaching tasks involving the shoulder and elbow.60 The Mitsubishi Pa 10-7 robotic end-effector arm (Mitsubishi S.P.A., Tokyo, Japan) was employed by Belfatto et al. for executing 3D point-to-point movements.54 The Gloreha Sinfonia (Idrogenet S.R.L., Brescia, Italy), as reported by Scotto di Luzio et al., is a hand glove exoskeleton, specifically designed for hand rehabilitation.53 Lastly, the Arm Light Exoskeleton Rehab Station (ALEx-RS) by Wearable Robotics srl, was utilized in the study by Pierella et al. for a 3D point-to-point reaching task using an upper-limb exoskeleton.52 Unlike the other devices, the Brain-Machine Interface (BMI) controlled robot used in the study by Irastorza-Landa et al. represents a novel experimental approach.56 All the devices offer a specific level of guidance when the subject is unable to reach a target within a specified time frame. In the study by Lencioni et al., the robotic system facilitated reaching movements in two distinct force modes: assist-as-needed and resistive chosen by the physiotherapist.60 The studies by Pierella et al. and Tropea et al. described robots that provide force as needed during therapy sessions.52, 55 Scotto di Luzio et al. used a hand exoskeleton in active-assisted mode during the rehabilitation treatment, where the non-paretic hand starts the movement and the paretic hand follows the same movement with assistance.53 In the study by Nerea Irastorza-Landa et al., both a hand orthosis and an arm orthosis were controlled via a BMI system, which allowed for the modulation of sensorimotor rhythms detected through EEG to control the movement of the orthoses.56 The movement of the orthoses was synchronized with the patients’ intentions to move, as detected by their brain activity.
Protocols for rehabilitation sessions
The rehabilitation protocols detailed in the analyzed studies showcase a diverse range of approaches, each tailored to enhance post-stroke recovery through the reorganization of muscle synergies. In the study by Pierella et al., participants engaged in a 3D point-to-point reaching task for 30 minutes per session, three times a week over four weeks, totaling 12 sessions.52 Belfatto et al. employed the robotic arm for executing 3D point-to-point reaching and hand-to-mouth movements. The therapy consisted of 12 sessions of robot-assisted movements (3 sessions per week), each one lasting 40 minutes.54 Tropea et al. implemented a protocol where patients performed movements in the eight cardinal directions, with each session lasting 45 minutes, five times a week for six weeks, resulting in 30 sessions.55 Scotto di Luzio et al. focused on hand rehabilitation, where patients performed four different grasps with a robotic glove, aiming to grab and hold objects like a can, pencil, sheet, and tennis ball for ten seconds, with a rest period of at least ten seconds between attempts.53 This protocol was conducted five times a week for four weeks, totaling 20 sessions. Lencioni et al. explored the effects of robot-assisted versus usual care rehabilitation, where participants in the robot group controlled the position of an end-effector in planar movements, guiding their paretic limb back and forth from a central position to five random targets on a circumference.60 This was done for 45 minutes per session, five times a week over four weeks, amounting to 20 sessions. Lastly, Irastorza-Landa et al. utilized a BMI controlled robot for hand and arm rehabilitation, where patients controlled a hand orthosis for finger flexion and extension and an arm orthosis for elbow movements.56 This intensive therapy was combined with 1 hour of conventional physiotherapy, with daily sessions lasting one hour, for a total of 17±1.8 days. Some patients additionally received arm-BMI training for 5.11±4.27 sessions.
Protocols for synergy extraction
The movements performed for synergy extraction in these studies varied, from different types of reaching to hand tasks. In Pierella et al., patients navigated a 3D spherical workspace with the device, aiming to reach as many of the 18 targets as possible within 30 minutes.52 Scotto di Luzio et al. recorded EMG data during 10 seconds of grasp and hold task using different objects in front of a screen that showed the task to perform.53 Tropea et al. developed a platform where patients executed reaching tasks in 8 cardinal directions on a horizontal plane in one session, contrasting with healthy participants who engaged in five sessions at distinct cadences.55 Belfatto et al. focused on the forward phases of bringing the hand towards the mouth moving a robotic handle through a predetermined trajectory without assistance.54 Lencioni et al. used two 3D motor tasks, the transport and placing task, to assess upper limb performance of both proximal and distal districts.54 Irastorza et al. involved 40-60 free bilateral movements across five directions in front of a screen following auditory and visual cues.56 The tasks were performed with both limbs in three studies.53, 56, 60 The baseline postures for the first six studies involved subjects seated in various configurations to perform specific tasks. In Pierella et al., subjects were seated with the upper body and arm positioned by an exoskeleton with the elbow bent.52 Scotto di Luzio et al. required subjects to sit, elbows bent at 90 degrees, forearms horizontal, and wrists neutral.53 Similarly, Tropea et al. had subjects seated, facing a monitor, with shoulders slightly abducted, elbows bent at 90 degrees, and forearms supported horizontally.55 Belfatto et al. described subjects in a seated position with relaxed, slightly abducted shoulders, extended elbows, and neutral wrists grasping a robotic arm.54 Irastorza-Landa et al. involved subjects seated with hands resting on their laps, performing bilateral upper limb movements.56 Lencioni et al. positioned subjects seated with their elbows at 90 degrees and hands initially on their thighs for the object placing task.60 For the pronation task, subjects were positioned with their elbows at 90 degrees, wrists fully supinated, and shoulders laterally rotated so that the forearms were approximately 45 degrees relative to the thighs. In both tasks, subjects grasped a VRRS electromagnetic sensor.60
The number of muscles examined ranging from 6 to 16 depending on the task. Scotto di Luzio et al. focused on 6 hand and finger muscles essential for grasping and manipulation tasks.53 Tropea et al. studied 10 key muscles for shoulder and elbow movements during reaching tasks.55 Belfatto et al. examined 8 muscles involved in shoulder and elbow motions.54 Irastorza-Landa et al. analyzed 11 muscles: essential for shoulder, elbow, wrist, and hand movements.56 Pierella et al. analyzed 15 muscles involved in shoulder, elbow, and forearm movements.52 Lencioni et al. studied 16 muscles, which are key muscles for shoulder, elbow, and forearm movements.60 Common muscles across studies included upper trapezius, anterior deltoid, middle deltoid, posterior deltoid, pectoralis major, biceps brachii, brachioradialis and triceps brachii. In addition pectoralis minor, infraspinatus, rhomboid major, brachialis, pronator teres, and supinator were also examined. Only Scotto di Luzio et al. involved flexor and extensor digitorum superficialis, flexor and abductor pollicis brevis, abductor and extensor digiti minimi.53
Algorithms for synergy extraction
In the analysis of muscle synergies from EMG data, Non-NMF emerged as the predominant algorithm, employed in five studies. Specifically, Pierella et al. utilized NMF, setting a VAF threshold of 95% to ensure a high fidelity in synergy extraction.52 Similarly, Irastorza-Landa et al. applied NMF with the same VAF threshold of 95% in their study.56 Scotto di Luzio et al. and Lencioni et al. both employed NMF but opted for an R2 value of 80% and 90% respectively, to assess the goodness of fit in their studies on hand muscle synergies and upper limb muscle synergies.53, 60 In contrast, Tropea et al. used FA with criteria based on eigenvalue greater than 1 and a cumulative variance slope below 75% of the shuffled dataset.55 Belfatto et al. also used NMF and VAF to explain at least 80% of the variance to evaluate synergies.54 The studies employed various methods for similarity analysis to evaluate muscle synergies. Pierella et al. assessed synergy similarity using the scalar product between synergies from patients and healthy subjects.52 Scotto di Luzio et al. compared synergies within the same patient across different conditions using Cosine Similarity (CS) and a Similarity Index (SI).53 Tropea et al. measured similarity using the scalar product normalized to the Euclidean norm for both intra-group and inter-group comparisons, and Pearson correlation coefficients for comparing temporal components.55 Belfatto et al. used scalar products to compare spatial components of synergies and Pearson’s correlation coefficient to compare temporal components before and after rehabilitation.54 Nerea Irastorza-Landa et al. employed the Functional Synergy Recruitment Index (FSRI) and indexes for preservation, merging, and fractionation of synergies between paretic and healthy limbs to assess similarity.56 Lencioni et al. conducted synergy comparisons using module similarity (scalar product) and activation profile similarity (Pearson’s correlation coefficient).60
Variation of the number of synergies in longitudinal treatments
Three studies examined the impact of robotic treatment on the number of muscle synergies.52, 56, 60
Pierella et al. observed an increase in the number of muscle synergies over time in subacute stroke patients,52 whereas Lencioni et al. found no significant changes compared to healthy controls.60 Irastorza et al. observed no significant changes in preservation, merging and fractionation indexes post-therapy. Three studies used a pre-determined number of synergies for their analysis.53-55 Tropea et al. used four synergies for all subjects, stating that this number was chosen as it was the minimum number of synergies able to account for at least 70% of the variance of the original EMG dataset in all subjects.55 Scotto di Luzio et al. set the number of synergies to three for all subjects and conditions, based on preliminary tests showing that three synergies could explain at least equal to 80% of the R2 in all subjects.53 Belfatto et al. used two synergies for all subjects, explaining at least 80% of the variance of the signal.54
Variation of synergies structure in longitudinal treatments
Scotto di Luzio et al. found that muscle patterns in the injured limb of chronic stroke patients became more similar to those in the healthy limb after the intervention.53 Similar findings were also described in subacute stroke patients compared to healthy controls following robotic intervention.52 Lencioni et al. observed that robot-assisted therapy significantly impacted muscle weighting in one of two synergies during an object placing task and robot-assisted and usual care intervention induced changes in both synergies in forearm pronation tasks.60 Conversely, Belfatto et al. observed that the robot training focused on hand-to-mouth movements did not produce any noticeable changes between pre and post-treatment evaluations in chronic stroke patients.54 Tropea et al. found that the robot-assisted training did not significantly affect similarity between healthy subjects and subacute stroke patients but restored intra-group similarity.55 Irastorza et al. found no significant difference in synergy structure between pre and post-treatment evaluations for healthy or paretic limbs.56
Variation of temporal coefficients in longitudinal treatments
Three studies addressed the temporal component analysis of muscle synergies. Tropea et al. observed that subacute stroke patients undergoing robot-assisted therapy exhibited more consistent activation patterns, aligning more closely with those observed in healthy individuals.55 Belfatto et al. observed that after robotic therapy, the temporal components of muscle synergies in post-stroke patients exhibited varying degrees of correlation.54 Specifically, while some patients showed positive correlations, indicating consistent synergy activation patterns before and after the intervention, others demonstrated negative correlations, suggesting a divergence in the temporal activation of synergies post-therapy. Lencioni et al. observed that robotic treatment significantly affected the temporal profile of one muscle synergy in post-stroke patients during an object-placing task, leading to an activation pattern that resembled those of healthy controls. However, both robotic and usual care interventions were found to alter the temporal profiles of muscle synergies during a forearm pronation task in a way that deviated further from the normative patterns, that authors considered as a negative effect on these specific muscle synergy activations.60 Irastorza et al. found differences only in temporal activations measured with functional synergy recruitment index.56
Clinical and instrumental findings
Pierella et al. found significant improvements in FMA-UL scores and grip strength across all assessments.52 Scotto di Luzio et al. reported that all patients showed a significant increase in the FMA-UL and Motor Power (MP) scores.59 Tropea et al. observed an average improvement in FMA-UL score of 72.8% between baseline and mid-training assessments. Patients also showed an improvement in the Motricity Index.55 No significant change was found in the MAS score. Belfatto et al. found no significant improvement in FMA-UL and WMFT scores.54 Irastorza-Landa et al. reported significant improvement in the combined and upper-arm subsections of the modified FMA.56 The hand/finger subsection showed a non-significant increase.56 No significant reduction was found in the level of spasticity measured by MAS post-intervention.56 Lencioni et al. found non-significant improvement in motor ability as measured by FMA-UL score in both the robot-assisted and usual care groups.60
Four studies incorporated kinematics to evaluate motor performance during specific tasks.52, 54, 55, 60 Belfatto et al. and Lencioni et al. utilized optoelectronic systems, to track movements.54, 60 Belfatto et al. examined the forward phase duration and specific joint angles, including those of the shoulder and elbow,55 while Lencioni et al. focused especially on articular angles such as elbow extension, wrist pronation, and others. Two studies derived their kinematic data from the devices themselves. such as mean tangential velocity, movement accuracy smoothness,52 number of peaks, movement smoothness, and hand path error.55
Lencioni et al. reported that the robot-assisted group showed better elbow extension and trunk movement in an object-placing task and in wrist pronation compared to the usual care group.60 Furthermore, the robot-assisted group had fewer smooth movements compared to usual-care group, which was associated with better performance. The usual care group exhibited shoulder angles during the forearm pronation task that were closer to those observed in healthy adults.60 In their study, Lencioni et al. observed that kinematic improvements in the robot-assisted group were associated with changes in the activation profile of muscle synergies. This was evidenced by a significant increase in the similarity of muscle weightings and activation profiles of synergy 2 of object placing task compared to the usual care group, suggesting that the kinematic improvements were directly linked to these alterations in the activation profile. Pierella and Belfatto found patients’ movement increased in speed and smoothness with a reduction of jerkiness and robotic assistance, although some variability among patients was found.52, 54
Two studies, also employed EEG to explore cortical activations and movement abnormalities during motor tasks. Belfatto showed that alterations in EEG-based parameters reflect alterations in muscle synergies structure, while kinematics showed only partial coherency with muscle synergies.54 Pierella in their multimodal analysis found correlations among EEG, kinematics and muscle synergies and clinical scales.52
Non-robotic studies
Enrolled patients and volunteers
The eight selected studies covered a diverse range of patient demographics and stroke characteristics.57-59, 61-64, 68 Similar to what was observed in robotic studies, authors have utilized varying subsets of the FMA domains to calculate the final score. The FMA-UL comprises 33 items for motor function (section A-D) for a cumulative score range from 0 to 66. Sensation (total score 12), passive joint motion (total score 24) and joint pain (total score 24) are considered separately. Only Maistrello et al. evaluated 50 subacute and chronic patients with a Fugl Meyer score of 117.20±24.57 at the baseline,58 suggesting that the authors may have used a modified or extended version of the Fugl-Meyer scale that includes additional items, or they have combined the upper and lower extremities scores for a total Fugl-Meyer score. Niu et al. studied six patients, five in the subacute and one in the chronic stage of stroke.57 At baseline, their motor impairment was described as a FMA-UL score ranging from 15-28.57 In their subsequent study, the group examined 16 ischemic stroke patients in both subacute and chronic phases, with most having left-side impairment ranging from low to severe.62 Hesam Shariati et al. studied 24 chronic ischemic and hemorrhagic stroke patients with mainly non-dominant side impairment, assessing baseline with FMA-UL scores averaging 46.7±3.6.63 Seo et al. examined 32 chronic patients with moderate to severe left-side upper limb impairment, scoring 8-40 on FMA-UL.64 Alnajjar et al. focused on 9 healthy and 10 subacute stroke patients.59 Their motor impairment was evaluated using the Stroke Impairment Assessment Set (SIAS) – knee-mouth task, scoring between 2-4 out of 5. Zendehbad et al. considered both 12 healthy and 24 subacute stroke patients, mostly with right-side impairment and an average FMA-UL of 21.66±7.37.61 Dash et al. included 8 healthy and 12 chronic stroke patients, focusing solely on residual grip function without detailing upper limb impairment.68
Types of intervention
Two studies used synergy-based functional electrical stimulation (FES).57, 62 FES involves applying electrical currents to stimulate muscles and facilitate motor tasks. Additionally, three studies utilized VR (VRRS®, Khymeia Group Ltd., Noventa Padovana, Italy;58 a VR-enabled sEMG-triggered grip exercise platform, Gripx system;68 Nintendo Wii, Nintendo, Japan63), one study used a myoelectric computer interface (Myo-CI) which involved a game developed in Python that interfaced with custom C++ software to acquire the EMGs (Trigno, Delsys, Inc.) from the targeted muscles.64 Alnajjar et al.’s study exclusively utilized conventional therapy.59 Meanwhile, in the research conducted by Zendehbad et al., a specific data collection device (Thought technology. Montreal, Canada) for a synergy-based visual biofeedback training was used.61
Protocols for rehabilitation sessions
These studies explored various rehabilitation protocols aimed at improving motor function in stroke patients. Niu et al. conducted two experiments to examine synergy-based FES effects stroke patients’ upper limb movements.57 The movement task was to extend the upper limb away from the trunk on a horizontal surface, reaching movements of 36 cm in forward direction or 48 cm in lateral direction. In Experiment 1, the participants performed 30 to 50 trials of forward and lateral reaching movements with FES assistance. In Experiment 2, the participants received daily FES interventions for five consecutive days along with conventional physical therapy.57 In their innovative approach to post-stroke rehabilitation, Hesam-Shariati et al. employed Wii-based games designed to encourage the use of the more affected upper limb in a largely unconstrained movement environment. The therapy protocol involved 1-hour sessions over 10 consecutive weekdays, augmented by prescribed home practice.63 Seo et al. explored the use of a myoelectric computer interface (MyoCI) training protocol to address abnormal muscle co-activation in chronic stroke survivors.64 The EMG signals from specific arm muscle pairs were used to control a cursor in a custom-designed computer game, thereby reducing abnormal co-activation by training participants to activate each muscle within the pair separately. The training was structured into three groups: one group trained isometrically for 60 minutes, another isometrically for 90 minutes, and a third group trained for 90 minutes with unrestricted limb movement. Maistrello et al. and Dash et al. involved patients performing specific sets of exercises during rehabilitation sessions, such as shoulder flexion, internal-external rotation, and hand-digit motion. Maistrello et al. conducted 20 sessions of 1 hour each, five sessions per week, for 4 weeks total.58 Dash et al. also incorporated sEMG-based biofeedback (6-7 weeks with 2-3 sessions/week) to improve training efficacy.68 Alnajjar et al. used a regular rehabilitation program, although the specifics were not described.59
Protocols for synergy extraction
Niu et al. in both of their studies involved reaching movements, with upper limb extensions away from the trunk on a horizontal surface. Participants were instructed to practice each movement 20-40 times before the experiment until they could comfortably accomplish the task.57, 62 In Hesam Shariati et al. the EMG of each muscle was averaged over 10 consecutive Wii-baseball swings for each patient,63 while Maistrello et al. used a VR rehabilitation system with unspecified motor tasks in a virtual environment.58 Dash et al. involved grasping and lifting a glass and moving it to another position while keeping the elbow resting on the armrest.68 Seo et al. focused on reaching to six targets placed at different heights, while Alnajjar et al. involved a simple bimanual shoulder flexion task with a set of 10 to 15 trials for each session.59, 64 Zendehbad et al. had participants sit on a chair, executing shoulder movements with internal and external rotations.61 In these studies, the baseline postures also involved subjects seated in various configurations. Niu et al. had subjects seated with the shoulder in a neutral to slightly abducted position, elbow flexed at 90 degrees, and wrist in a neutral position.57, 62 Hesam Shariati et al. involved therapy performed standing whenever possible, with some patients starting seated if necessary, ensuring time spent standing was progressively increased.63 Dash et al. had subjects seated upright with upper arms hanging vertically, elbows bent at 90 degrees, forearms horizontal, and wrists neutral.68 Seo et al. involved subjects seated upright with a slight shoulder elevation, elbow bent at 90 degrees, and forearm extended forward horizontally.64 Alnajarr et al. had subjects seated with the shoulder abducted at 90 degrees, elbow extended, and forearm in a neutral position while holding a robotic manipulandum.59 Zendehbad et al. involved subjects seated with shoulders flexed forward, elbows extended, forearms parallel to the ground or slightly sloping downward, and wrists in a neutral position.61 Maistrello et al. did not specify a baseline posture.58
The studies examined 4 to 16 muscles, focusing primarily on key muscles of the shoulder (e.g., deltoids, trapezius, pectoralis major),57-59, 61, 62, 64 upper arm (e.g., biceps brachii, triceps brachii, brachialis),57-59, 62-64 forearm (e.g., brachioradialis, pronator teres, supinator, wrist flexors and extensors)58, 63, 68 and fingers (e.g. finger flexors and extensors).68 Niu et al. analyzed 7 muscles.57, 62 Hesam Shariati et al. studied 6 muscles.63 Maistrello et al. examined 16 muscles.58 Dash et al. and Zendehbad et al. analyzed 4 muscles.61, 68 Seo et al. studied 8 muscles.64 Alnajarr et al. examined 5 muscles.59
Algorithms of synergies extractions
The methods for selecting the optimal number of synergies and their weighting varied across studies. Hesam Shariati et al. used NMF with a VAF threshold of 97%.63 Alnajarr et al., Seo et al. and Maistrello et al. used NMF with a VAF threshold of 90%.58, 59, 64 Zendehbad et al. used Hierarchical Alternating Least Squares (HALS) with an R2 threshold of 0.9.61 Niu et al. used NMF with a VAF threshold, but the specific threshold value was not reported.57, 62 Dash et al. did not report further details.68 The studies employed various similarity methods to analyze muscle synergies. Niu et al. in their studies used similarity of synergy vectors (SV), similarity of time profiles (ST), and a combined similarity index (SCOM) to evaluate changes before and after treatment.57, 62 Hesam Shariati et al. utilized the scalar product of synergy timing profiles to compare patients with similar motor functions.63 Maistrello et al. assessed similarity through the number of synergies in affected and unaffected limbs, scalar products between synergies, and median scalar products, along with the mean number of unaffected synergies merging into each affected synergy.58 Dash et al. computed the Synergy Stability Index (SSI) to assess the consistency of synergies before and after training.68 Seo et al. computed the disparity index to compare synergy weights before and after training.64 Alnajarr et al. and Zendehbad et al. did not specify additional similarity methods.59, 61
Variation of the number of synergies in longitudinal treatments
These studies found no significant post-intervention changes in the number of muscle synergies. Only Hesam Sheriati et al. noted a non-significant increase for patients with low to moderate motor function.63
Variation of synergies structure in longitudinal treatments
The examined papers reported changes in muscle synergy composition post-treatment. Among these studies, similarity parameters were frequently employed to measure changes. Seo et al., utilizing the “Disparity Index”, identified notable intra-individual changes in the number and composition of synergies in chronic stroke patients, without discerning a general trend in these parameters.64 The Disparity Index increased after the training in the participants who responded to Myo-CI training. This trend was not observed in participants who did not respond to the training. Dash et al. reported a significant increase in SSI during a glass-lifting task, although the specific components constituting these muscle synergies were not detailed.68 Maistrello et al. focused on the median of the scalar product between the affected and unaffected upper limb (Median-sp) with no significant change after the intervention.58
Variation of temporal coefficients in longitudinal treatments
In both studies, Niu et al. analyzed temporal components.57, 62 They observed significant time profile changes in all three muscle synergies post-FES in both subacute and chronic patients during forward and lateral reaching.
Hesam Sheriati et al. measured temporal components solely to distinguish motor function levels, without reporting post-treatment changes.63 Other papers did not provide temporal coefficient variations.
Clinical outcomes and correlated findings
Most studies indicated clinical improvement following interventions. In Niu et al., it was revealed that 5-day of synergy-based FES. FMA-UL increased for all three patients (an increase of 6, 8, and 3 points respectively).57 The same group in 2022 demonstrated that after a five-day intervention of synergy-based FES, FMA-UL scores increased significantly by 6.67±5.20 points in the FES group compared to a 2.00±2.38 point increase in the Sham group.62 Hesam Shariati et al. indicated improvement for WMFT timed tasks (WMFT-tt), FMA, and Motor Activity Log Quality of movement (MALQOM) over time.63 Clinical assessments and game performance demonstrated improved motor function for all patients at post-therapy, and these improvements were sustained at 6-month follow-up. Ashworth scores at the wrist, elbow, or shoulder did not change post-therapy. Maistrello et al. found that the FMA score improved by 6%, and the RPS score by 4%. A significant rise in FMA-UL scores from baseline was recorded at the 6-week checkpoint in Seo et al. study.64 Zendehbad et al. reported a statistically significant increase in both groups in terms of the FMA-UL, National Institutes of Health Stroke Scale (NIHSS) and Modified Rankin Scale (MRS) score. In contrast, Alnajjar et al. did not find significant motor function improvements using the SIAS.59 Dash et al. observed improved grip strength and pinch force in stroke patients using a dynamometer.68
Maistrello et al. identified significant correlations between certain clinical scales and post-treatment muscle synergy parameters.58 Specifically, the Modified Ashworth Scale (MAS) was correlated with the number of affected synergies. Both the FMA-UL and the RPS were correlated with the percentage of synergies shared in the affected arm (Nsh-naff) and Median-sp.
Niu et al. investigated kinematics and joint angle trajectories to assess motor recovery after stroke resulting in an increased peak velocity in reaching movement after FES treatment.57, 62 They reported a correlation between changes of elbow flexion and the changes of synergy similarity, but only in the FES group. The data for the population of Seo et al. study derived from previous published data showed improvement in the range of motion of the elbow.66
Discussion
This scoping review examines robotic and non-robotic treatments’ impact on upper limb muscle synergies in stroke patients. The following sections will provide a critical analysis of the review’s findings, elucidating the significance and implications of muscle synergies in the interventional studies for stroke rehabilitation. While our emphasis has been on robotic studies, we also considered non-robotic treatments to provide a comprehensive perspective.
Muscle synergies in robotic stroke rehabilitation
Over the years, many devices have been developed and tested for upper limb rehabilitation, ranging from simple mechanical aids to sophisticated robotic systems. In the literature, more than a hundred devices have been described for upper limb rehabilitation.69 While the studies showed promising results in terms of the potential benefits of technology-aided rehabilitation,70 it is still unclear how long these benefits last and how they translate to functional improvements in daily life,71 and what neural mechanisms are associated to motor recovery. Within this context, muscle synergies present a promising avenue.
Enrolled patients and volunteers
Significant patient heterogeneity was found in the included studies. In fact, there was no uniformity in the stroke stage, stroke type, and paretic side among the patients included. Additionally, the sample size in some studies was typical of pilot studies and not sufficient for strong statistical evidence, and the population in terms of motor impairment, affected side, and type of lesion was not precisely defined or characterized.
The studies included focused on subacute stroke patients and chronic stroke patients. The period following a stroke is categorized into distinct phases based on the understanding that recovery processes after a stroke are time-sensitive.72 Although there is some variability and the process is complex and non-linear, typically, recovery peaks are reached in the first weeks and approach a plateau after three months.73 By six months, the deficit is essentially constant, but patients can still experience gains with specific training or therapies.74 It is crucial to account for the “time after event” variable to ensure that the findings are not only accurate but also related to the specific stage of recovery, thereby allowing for more tailored and effective therapeutic strategies.
The level of motor impairment is another key parameter influencing study outcomes. Motor impairment can vary widely among stroke survivors, ranging from mild deficits to severe. This variability in motor function can impact the trajectory of recovery and the response to therapeutic interventions.39 The heterogeneity in motor impairment in the reviewed studies could potentially influence the interpretation of functional outcomes following rehabilitation interventions. Furthermore, the degree of motor impairment could influence the number of muscle synergies that can be identified in a patient.63 This might be due to the loss of independent control of muscles, leading to a different organization of the muscle coordination patterns.35 According to Tropea et al., the improvements of motor performance across patients occur in accordance with their own clinical picture, which is characterized by great inter-subject variability.55 This variability is due to the different recovering mechanisms resulting from the heterogeneity of sides, location and dimension of the brain lesion. Therefore, the authors suggest that a greater number of participants can provide further evidence for this result and that all meaningful data are reported.55 Additionally, the authors recommend that the analysis of the correlation between motor outcome and muscle synergies should be carried out for each individual patient in order to avoid bias due to the inherent inter-patient’s variability.55 Lencioni et al. also acknowledge the issue of inter-subject variability resulting from the heterogeneity of stroke pathogenesis, which could involve different mechanisms of recovery.60 To address this challenge, the study employed a prospective, randomized, multicentric, and single-blinded trial design, which helped minimize bias and manage the variability among post-stroke subjects. By adopting a prospective approach ensuring consistency in how the interventions were administered and outcomes measured. Randomization ensured that both treatment groups were balanced in terms of participant characteristics, neutralizing potential confounding factors. The multicentric nature of the study increased the sample size and provided a diverse patient pool, which improved the generalizability of the results. Finally, the single-blinded design reduces observation bias. Together, these design elements helped manage the heterogeneity among the subjects.
Three of the reviewed studies incorporated healthy control groups, while three studies utilized only the ipsilesional limb as a control.52, 55, 60 However, the use of the ipsilesional upper limb as a control group in the investigation of muscle synergies post-stroke has been questioned as the ipsilesional upper limb may exhibit altered motor patterns due to compensatory mechanisms engaged by the remaining motor functions.60 Consequently, these patterns may not always reflect the motor performance of a healthy individual. Furthermore, the authors discussed the importance of considering the bilateral hemispheric control of the distal upper limb when planning rehabilitation strategies.60 This is crucial as the ipsilesional upper limb may also be affected by the stroke.75 Therefore, using the ipsilesional upper limb as a control may not provide a precise comparison of motor performance as the comparison between stroke and healthy individuals. This highlights the need for careful selection of control groups in studies investigating muscle synergies post-stroke. Together, these factors emphasize the need for careful consideration in interpreting and comparing findings across different studies, ensuring that all patients characteristics are considered.
Types of interventions
The studies included employed a range of robotic devices, each with its unique design and purpose. These devices target motor tasks associated with the upper limb, encompassing the shoulder, elbow, wrist, and hand. The ALEx RS52 and Mitsubishi Pa 10-754 stand out for their ability to facilitate 3D upper limb movements, offering a more comprehensive range of motion. In contrast, devices like the InMotion255 are specialized for specific tasks, such as horizontal plane reaching, while the Gloreha Sinfonia53 is designed for hand-specific tasks like grasping and pinching. Morasso et al. emphasized that effective rehabilitation robots should have haptic properties and an adaptive, assist-as-needed approach.76 They also highlighted the importance of a high mechanical compliance, and the need for having a robot with low-stiffness control.76 They proposed a framework for addressing optimal assistance and learning paradigms without eliminating kinematic errors during the treatment.76 Indeed, error-based learning is a critical component of motor learning, particularly within the context of neurorehabilitation.77 This form of learning arises from the active engagement of the patient and the ability to adjust motor strategies based on experienced errors.78 It has been suggested that such error experiences provide essential information that aid in refining subsequent movements.79 This concept is encapsulated in the “guidance hypothesis” in motor learning, which proposes that over-guidance during the learning process can hinder motor learning, as it reduces the learner’s active problem-solving involvement.80 Research by Milot et al.81 and Marchal-Crespo et al.82 supports this idea, with their findings indicating that in robot-assisted rehabilitation, a balance between providing sufficient support for task completion, without overshadowing the patient’s active engagement and error-based learning, is essential for improved motor learning outcomes. In the context of muscle synergies, this aspect has been partially explored. Cancrini et al. conducted a comprehensive study on the effects of robotic assistance on upper limb spatial muscle synergies in healthy individuals during planar upper limb training exploring the guidelines and modes of assistance for robotic treatment in rehabilitation.83 In this context, the “standard assistance paradigm” refers to a force tunnel assistance approach, where the robot provides dynamic support along a predefined trajectory, offering corrective forces only when the user deviates from the path. This method is designed to minimally interfere with the natural movement, thereby preserving the number and structure of involved muscle synergies.83 Thus, the authors favored the use of robotic assistance paradigms in rehabilitation, but also emphasized the need for further scientific investigation into the impact of robot assistance on motor control.83 Additionally, Jarrassé et al. emphasized the importance of device transparency, the ability of a robotic device to follow human movements without applying any noticeable resistance or force advocating for robot intervention only when necessary to ensure correct motion and prevent frustration.84 Lack of detailed information on the mode of assistance underscores the necessity for thorough reporting in future research.
Protocols for rehabilitation sessions
The reviewed studies showcased a diverse range of intervention protocols in terms of duration, frequency, and intensity, reflecting the multifaceted nature of robotic-assisted rehabilitation. Most interventions were structured around three to five sessions weekly (4.33±0.94), each lasting between 30 minutes to an hour (44.0±9.7) culminating in a total of 12 to 30 sessions (18.5±6.5) over a span of three to six weeks (4.33 ±0.75). This observed variability in robotic-assisted stroke rehabilitation protocols across studies can be attributed to the absence of universally accepted and standardized guidelines governing the application of robotic interventions in post-stroke care.85
Central to all robotic rehabilitation approaches is the objective of enhancing motor capabilities in stroke survivors via consistent, methodical exercises.86 A key feature of these interventions is movement variability, where patients are exposed to diverse and challenging motions using robotic devices to enhance recovery. This variability helps restore generalized motor programs, leading to a partial recovery of normal muscle synergies or more efficient use of remaining ones.10 Incorporating variability, is crucial when applying synergistic approaches in robotic rehabilitation.87 Reviewed studies have demonstrated the benefits of incorporating movement variability in robotic rehabilitation. Tropea et al. asked patients to move a handle in eight directions, requiring different reaching movements.55 Lencioni et al. focused on planar motion using point-to-point reaching tasks, where patients had to move a handle or end-effector to reach random targets on a circumference.60 Whilst multi-directional planar setups can be effective in detecting synergy modulations across various directions, they may not fully encompass the clinical implications for patients, given that these setups many not consider the limb’s elevation against gravity and the coordination required for 3D tasks.88 For instance, Pierella et al. used an upper limb exoskeleton to guide patients in a 3D point-to-point reaching task, where they had to move between targets with visual feedback and assistance.52 Accordingly, Belfatto et al. used an end-effector device for 3D point-to-point reaching task.54 The importance of 3D movements in assessing and rehabilitating motor control cannot be overstated, as they offer a more thorough depiction of the human body’s natural and functional range of motion.89
Future work could improve muscle synergy analysis by maintaining motor variability and incorporating 3D movements.87 While current rehabilitation protocols often focus on simple and repetitive movements in one plane, introducing more complex and multi-planar movements can help to better represent the natural variability of human motion90 and to stimulate neuroplasticity and motor learning.91
Protocols for synergy extraction
In robotic-assisted rehabilitation and muscle synergy analysis, movement types and extraction methodologies are crucial. The examination of synergy extraction protocols reveals a diverse range of methodologies employed across various studies, with the types of movements performed and the number of muscles examined varying significantly. On the positive side, the broad array of tasks, ranging from different types of reaching to hand manipulation, ensures a comprehensive analysis of muscle synergies, as shown in recent comprehensive spatial mappings of muscle synergies in highly variable upper limb movements of healthy subjects.87 Moreover, the flexibility in the number of muscles examined allows researchers to tailor their studies based on the specific research question, available resources, and the population under investigation.
Distinguishing muscle synergies in device-assisted and free movements is essential for understanding real-world motor behavior. Device-assisted tasks provide the precision and control needed for targeted rehabilitative strategies, while free movements contribute to ecological validity. Ideally, a comprehensive evaluation would incorporate both types of movements to fully capture the complexity and adaptability of muscle synergies. Furthermore, tasks involving bilateral movements of the upper limbs56 can shed light on the interplay between the affected and unaffected limbs. Such tasks can also help in assessing how muscle synergies adapt or compensate when both limbs are engaged, which is crucial for tasks requiring bimanual coordination control.92 A point to provide reliable muscle synergies extraction relies on sources of variability within the same session and across sessions and subjects. A recent study explored the variability of muscle synergies in hand grasps, focusing on inter-session and intra-session variability.93 The researchers found that both types of variability significantly impact the stability and composition of muscle synergies. Inter-session variability, influenced by factors such as natural movement variability and differences in electrode positioning, can lead to considerable variation in muscle synergy composition between acquisition sessions.93 Intra-session variability, on the other hand, refers to trial-by-trial variability within the same session due to lower repeatability and larger heterogeneity in the movements of neurological patients compared to healthy individuals.93 Understanding variability is key for reliable synergy assessments and insights into muscle control and recovery mechanisms. To reduce variability in synergy-based assessments, several strategies can be implemented. Firstly, ensuring standardized electrode positioning is crucial for consistent and accurate placement of EMG electrodes across different acquisition sessions. Secondly, providing subjects with thorough training and familiarization with the experimental tasks can decrease natural variability in performed movements. Furthermore, maintaining a consistent baseline posture across sessions is essential for reducing variability in muscle synergy extraction. A standardized posture minimizes the effects of postural differences on muscle activation patterns, ensuring that any observed changes in synergies are due to the tasks or interventions rather than initial positioning. Additionally, careful session scheduling, considering factors such as fatigue and time of day, can help minimize variability by ensuring subjects are in a similar physiological state during each session. Fatigue can be a confounding factor that affects the reliability of muscle synergy data. To address this issue, a recent study used electromyogram-based indicators to adapt the task difficulty in robot-mediated upper limb training based on muscle fatigue.94 The authors proposed an algorithm that automatically detects fatigue based on EMG features, and changed the task difficulty to help participants avoid or delay fatigue. Lastly, employing robust synergy extraction algorithms that can handle variations in muscle activation patterns and adapt to different levels of variability across sessions is essential. Moreover, the diversity in tasks adds a layer of complexity to data analysis and interpretation. One variable that requires careful consideration is the number of repetitions in the choice of the right protocol. A minimum number of repetitions for the acquisition and extraction of muscle synergies is essential to guarantee a correct mathematical model. Future research should address both task-specific muscle synergies and adaptable training protocols, considering fatigue and repetition count.
Algorithms for synergies extraction
As a mathematical model, muscle synergy analysis involves numerous parameters that may influence the outcomes and interpretation of results. It provides several opportunities of interpretation of the data, but this complexity can pose challenges when attempting to replicate analyses across different research groups.12 In robotic studies, NMF is the most widely used method for extracting synergies from EMG data, with only one study from those selected in this scoping review, employing Factor analysis instead.55 Many models have been proposed in the literature to extract synergies, but most of them have not been exploited in this context. Some examples of mostly unused algorithms include the temporal model with NMF95 the spatiotemporal synergies,13 mixed matrix factorization (MMF),96 space by time decomposition,97 and autoencoder.98 Each algorithm has specific features that investigate peculiar aspects of motor control. Alessandro et al. argued that muscle synergy extraction methods should incorporate task execution variables, in order to link muscle synergies to the motor function they produce to better reflect task-space dynamics (such as joint angles or forces) and improve the interpretation of the results and their clinical use.29 They suggested to consider task-related synergy extraction method instead of NMF. To overcome these limits, Scano et al. proposed a novel factorization algorithm called mixed matrix factorization (MMF) that allows the extraction of combined “kinematic-muscular synergies” from data generated by non-negative combinations of known kinematic-muscular synergies.96 The proposed approach can provide insights into the functional role of motor modules and the relationship between muscle synergies and the motor output at the kinematic or kinetic level. O’Reilly and Delis proposed a computational approach that removes linearity constraints and links muscle synergies to the task space.99 Despite the variety of available models, all examined studies extracted and analyzed spatial synergies. This kind of synergy seems to explain motor coordination at a spinal level.17, 100 Studies in vertebrate species suggests that muscle synergies might be encoded by neural circuitry at both the spinal and cortical levels. In the frog spinal cord, N-methyl-D-aspartate (NMDA) iontophoresis, microstimulation, and cutaneous stimulation, evoked EMG patterns that could be decomposed into a small number of synergies similar to those seen in natural behaviors, implying the synergies are encoded by spinal interneuronal networks.19, 101-103 Hart and Giszter further demonstrated that the activity of spinal interneurons in the frog was more closely related to the activation of muscle synergies than to the activity of individual muscles.104 Recent work by Takei et al. provided evidence that spinal premotor interneurons in the primate cervical cord also underlie the spatiotemporal patterns of hand muscle synergies during a precision grip task.105 They found that the output effects of spinal interneurons on hand muscles were not uniformly distributed but rather clustered into groups corresponding to muscle synergies. More recently, York et al. developed a computational model of spinal circuits that could reproduce muscle synergy patterns significantly influenced by limb position and angles in humans.106 Their model suggests that proprioceptive afferent inputs to spinal interneurons can modulate the activations of agonist and antagonist muscle synergies encoded in the spinal circuitry. However, the motor cortex also likely plays a role in regulating and combining synergies. In monkeys, intracortical microstimulation of the primary motor cortex activated muscle synergies resembling those identified in natural reach and grasp movements.107 Furthermore, in stroke patients, alterations in muscle synergies were correlated with the extent of damage to the motor cortex.35 A conceptual model proposed by Drew et al. postulates that distinct motor cortical neuronal subpopulations control different synergies, allowing flexible recombination of synergies to produce diverse movements.108
Indeed, different types of synergy models may explain motor control at different levels of the CNS and introduce relevant updates that might explain hidden mechanisms of recovery that cannot be found with the standard spatial synergy model based on NMF.
Variation of the Number of synergies in longitudinal treatments
The number of muscle synergies depends on the number of muscles examined and thresholds of extracted synergies. To assess the quality of the extracted synergies, two metrics have been commonly used: the VAF and R2. They have different sensitivity and, given a threshold-based synergy number selection criterium, they can lead to a different number of extracted synergies.21, 109 Other methods are available, such as the linear fit110 and Akaike information criterion (AIC).111 A recent suggestion and operative method should be to use different thresholds associated to the VAF or R2 curves to provide a multi-resolution analysis, as introduced in Pale et al.93 Some simulation studies already found the importance of a correct number of muscles to analyze muscle synergies because they impact on synergy extraction and VAF can be overestimated by a small number of muscles analyzed.112 These studies suggested that a large set of muscle should be examined to avoid underestimation of motor control.113 Steele et al. suggested two methods to reduce the sensitivity of synergy analyses to the number and choice of muscles: selecting dominant muscles from a master set of synergies and selecting muscles with the largest maximum isometric force.112 The choice of muscles examined can significantly influence the extraction of synergies and may lead to overestimation of the VAF when only a few muscles are considered112, 113 or to misinterpretation of the level of disability. According to these observations, Pierella et al. analyzing 16 key muscles for a reaching task, were unique in reporting significant differences in the number of synergies after robotic treatment.52 Conversely, Lencioni et al. analyzed the same number of muscles, but they did not find any changes in synergy number.60 Scotto di Luzio et al. observed that the weighting of paretic muscle synergies became more similar to that of a healthy limb during grasping.53 However, they admitted that their results are not generalizable due to their small sample size and the limited number of muscles analyzed. Moreover, synergy decomposition procedures only care about explaining as much variance of muscle activity as possible, ignoring functional significance.26
Variation of synergies structure in longitudinal treatments
The studies reviewed provide compelling evidence that robotic-assisted therapy can significantly impact motor coordination in stroke patients, as evidenced by changes in muscle synergy structure post-intervention. Scotto di Luzio et al. in their study involving subacute stroke patients, demonstrated that robotic rehabilitation can potentially facilitate motor function recovery to a degree comparable to that of healthy individuals, as evidenced by a higher similarity between muscle patterns in the injured and healthy limb post-intervention.53 This aligns with the findings of Hashiguchi et al., who focused on the changes in muscle synergies during the recovery process, noting that the number of muscle synergies in stroke patients increased with recovery and became more similar to those of healthy individuals.114 This suggests that the recovery process involves a reorganization of motor control strategies, which could potentially be facilitated with robotic-assisted therapy. Furthermore, Lencioni et al. observed a significant treatment effect on contralesional upper limb muscle weighting in the robot-assisted group during an object placing task.60 This suggests that robotic interventions may enhance motor control and muscular coordination, improving task-specific motor functions. On the contrary, Belfatto et al. reported no significant change in hand-to-mouth movement between pre and post robotic treatment in chronic stroke patients.54 This is likely due to the fact that different tasks and robotic assistance can potentially affect the spatial structure of muscle synergy.115
Tropea et al. observed that while robot-assisted training did not significantly affect the similarity of muscle synergies between healthy subjects and subacute stroke patients, it did restore intra-group similarity in terms of muscle synergies in post-stroke patients.55 Lastly, Irastorza et al. found no significant difference in synergy structure between pre- and post-treatment evaluations for healthy or paretic limbs.56 These diverse findings underline the importance of using muscle synergies in the evaluation of robotic rehabilitation outcomes and highlight the need for further research to understand the specific impacts of these interventions on synergy structures and to develop more effective, patient-specific rehabilitation protocols.
Variation of temporal coefficients in longitudinal treatments
In these studies, we found that the temporal component of muscle synergies seems to be the most sensitive parameter that can be changed with rehabilitation treatment. In fact, the temporal component showed more changes than the synergy structure. However, only four studies reported the analysis of this parameter.54-56, 60 Temporal coefficients are often overlooked in synergy studies, which can be a limiting factor as highlighted in Zhao et al.88 Regarding the temporal synergistic model, Brambilla et al. investigated the differences in reconstruction accuracy between temporal and spatial synergy models across various upper limb movements. Their findings indicate that for achieving a specific level of R2, fewer temporal synergies are needed compared to spatial synergies. The research suggests that the temporal dimensionality of data may be more constrained by its smoothness than the spatial dimensionality is by the amplitude distribution.17 Tropea et al. observed that the alteration of the temporal component of muscle synergies reflects the injury of the cerebrovascular accident and could document the effects of the functional recovery due to a suitable and customized treatment.55 Lencioni et al. found that the similarity of temporal coefficient in object placing increased after robotic treatment and this change was associated with better kinematics. They attributed this effect to a true neurological recovery (as opposed to motor compensation). In fact, according to Tropea et al.’s study, the authors hypothesized that the abnormal recruitment of motor modules were the main cause of the impaired motor performance.60 Similar effects were also found in the study by Belfatto et al.54 Analyzing temporal coefficients is crucial for understanding muscle activation timing and recruitment changes. According to a recent review, temporal coefficients should be considered as biomarkers for detecting motor impairment.88
Clinical and instrumental findings
Multi-modal approaches in rehabilitation enrich our understanding of pathophysiological changes by revealing novel variable relationships.116 In this scoping review we focused on clinical and instrumental outcomes and their relationship with muscle synergies.
The FMA-UL was the primary outcome measure for motor impairment in most studies, showing improvement across stroke stages, except in one study.54 Cheung et al. highlighted the intrinsic relationship between the FMA and neural adaptations influencing motor recovery.35 In patients with mild impairment, indicated by higher FMA scores, there is a preservation of muscle synergies in the stroke-affected upper limb, similar to those in the unaffected upper limb. This phenomenon suggests that, despite observable motor performance variations, the foundational neural control mechanisms in these individuals remain largely conserved. As the severity of impairment increased, a divergence emerged between the muscle synergies of the affected and unaffected upper limbs. Cheung et al. identified certain muscle synergies in the affected upper limb that could not be solely attributed to synergy merging. Instead, these synergies seemed to represent fractionations of those in the unaffected upper limb.35
Clinical improvements often correlated with changes in the number, structure and timing profile of muscle synergies.52, 56 Irastorza et al. introduced the FSRI, which measures the temporal correlation between the recruitment profiles of healthy muscle modules in both affected and unaffected muscles. FSRI correlates with upper limb motor function and recovery scores, suggesting that functional synergy structures are physically preserved after stroke and authors proposed it as a biomarker to evaluate neuromuscular behavior and design motor personalized rehabilitation approaches.56 When combined with traditional clinical scales, muscle synergies can offer a more comprehensive picture of a patient’s motor function. This can provide insights into compensatory strategies adopted by stroke patients and guide interventions to restore normal movement patterns.
Beyond the relationship between synergies and clinical outcomes, some studies found correlations between kinematic and EEG parameters with synergy activation coefficients.52, 54 Kinematic analysis captures this variability in movement patterns, leading to insights into how different synergies are used by different individuals.117 Kinematic parameters can be acquired using various methods, such as marker-based optoelectronic systems or marker-less systems, like RGB-depth cameras or Inertial Measurement Units. The choice of method may depend on factors like accuracy, precision, ease of use, cost, and time required for measurements. Four studies included kinematic parameters in the evaluation. Belfatto et al. and Lencioni et al. used marker-based optoelectronic systems in their research studies,54, 60 while Pierella et al. and Tropea et al. extracted parameters from their chosen devices.52, 55 Indeed, upper limb rehabilitation robots are not only useful for providing therapeutic interventions, but also for assessing patients’ motor functions objectively.118
EEG is a valuable tool for studying motor control in neurorehabilitation. It can measure brain activity related to movement planning and execution, as well as the effects of external stimulation or feedback on motor cortex rhythms. The findings from two included studies indicate that robotic training can enhance cortical activity, bringing it closer to physiological conditions.52, 54 A recent paper demonstrated that finger movement induced by external devices had a significant effect on the mu-rhythm.119 EEG and EMG together may assess neural-muscular interactions.120 These insights are essential for advancing the field of neurorehabilitation, as EEG can be used as a non-invasive interface to control computers121 and robots, as demonstrated by Irastorza et al.56
Muscle synergies in non-robotic stroke rehabilitation
This section discusses muscle synergies in non-robotic stroke rehabilitation, building on longitudinal studies to highlight the potential for stroke recovery.
The eight studies comprised, subacute,57, 58, 62 chronic63, 64, 68 and mixed59, 61 stroke patients, with varying degrees of motor impairment. The inclusion of both healthy individuals and stroke patients, as seen in Dash et al. and Zendehbad et al., added another layer of variability to the findings.61, 68
While some studies, such as Niu et al., provided detailed assessments using the FMA-UL to gauge motor impairment, others, like Dash et al., did not specify the level of impairment. The studies reported a diverse array of outcomes, including functional scores like the FMA-UL, which was used in several studies to assess motor impairment and recovery.57, 61, 62, 64, 68 The WMFT was employed by Hesam-Shariati et al. and Seo et al. to evaluate upper limb function and movement quality.63, 64 The MAL was utilized by Hesam-Shariati et al. to assess the amount and quality of arm use in daily activities.63 In addition to clinical assessments, instrumental analyses were also performed. Kinematic parameters were evaluated in studies by Niu et al. and Seo et al. to quantify movement characteristics such as velocity, smoothness, and joint angles.57, 62, 64 EEG was employed by Zendehbad et al. to investigate cortical activity during motor tasks.61 This variety in patient characteristics and outcome measures reflects the heterogeneous nature of stroke rehabilitation research, highlighting the need for a comprehensive and multi-modal approach to assess motor function and recovery in stroke survivors. The treatment for studies comprised a mean duration of 50.8±13.2 minutes per session, and the mean number of sessions was 15.0±6.7. The mean duration of the treatment was 4.49±3.22 weeks.
Studies employed diverse interventions, from traditional rehabilitation therapies to modern techniques like FES, Myo-CI and virtual reality. This variety supports the widespread applicability of muscle synergy analysis in numerous rehabilitation procedures, aligning with the current trend in stroke rehabilitation that favors individualized, patient-centered care.122
As in robotic studies, the majority of the examined studies consistently employed NMF to decipher muscle synergies. This uniformity underscores the acceptance of NMF as a reliable technique for identifying intrinsic patterns of muscle coordination, which could guide the formulation of rehabilitation strategies. Nonetheless, the specific parameters employed, such as the VAF, demonstrated variation across the studies. The optimal choice for the VAF threshold, which influences the number of synergies discerned, remains disputed.123
The studies reported a substantial variation in the number of identified synergies, ranging from two to eight. Niu et al. fixed three synergies a priori,57, 62 Dash et al. used two fixed synergies68 and Zendehbad et al. used three fixed muscle synergies.61 Hesam-Shariati et al. found a significant difference in the number of muscle synergies between low motor-function (3.38±0.2) and high motor-function (4.00±0.3) groups at early therapy, with a non-significant increase for low and moderate motor-function groups at late therapy. Maistrello et al. reported that the number of synergies (8) did not change significantly after treatment.58 Seo et al. found no change observed after training.64 Alnajjar et al. identified one synergy in the affected upper limb before and after treatment, with notable changes in the level of VAF resembling the formation of two synergies.59 This disparity in the number of identified synergies could arise due to dissimilar task demands, the specific muscles analyzed, or participant demographics across the different studies. Existing literature corroborates that the complexity of tasks112 and patient-specific factors such as stroke severity and chronicity can modulate the number of synergies.10, 124 This broad variability underscores the need to determine an ‘optimal’ number of synergies based on the task or patient population, which could inform personalized rehabilitation strategies.
Interestingly, while some studies observed stability in synergy composition post-intervention, others reported alterations in the temporal components of the synergies.
In their two studies, Niu et al. specifically reported alterations in the temporal coefficients of muscle synergies following treatment.57, 62 In their 2019 study, they observed significant time profile changes in all three muscle synergies post-FES, suggesting that synergy-based FES might have incurred beneficial changes in muscle synergy.57 Similarly, in their 2022 study, Niu et al. found that individual data showed increased similarity than healthy controls in muscle vector and time profile, in forward and lateral reaching movements after treatment.62 The changes in temporal coefficients may reflect a reorganization of neural control strategies following FES treatment. This reorganization could involve more efficient recruitment of motor modules, resulting in better-coordinated muscle activations during specific movements, such as forward-reaching.
Hesam-Shariati et al. measured temporal components solely to distinguish motor function levels, without reporting post-treatment changes.63 Maistrello et al., Dash et al., Seo et al., and Alnajjar et al. did not provide any information on temporal coefficient variations following their respective interventions.58, 59, 64, 68 According to Dash et al., there was a notable increasing in the “Synergy Stability Index” during a glass-lifting task.68 This find could suggests that muscle synergy analysis could be a sensitive tool for detecting changes in motor coordination during goal-oriented movements. Hesam-Shariati et al. found that muscle weightings changed from early to late therapy except for the first four clusters, but differences pre and post treatment were not reported.63 Zendehbad et al. did not report any findings related to changes in synergy composition or temporal components.61
These findings highlight the need for more comprehensive reporting of muscle synergy parameters, particularly temporal coefficients, to better understand the effects of various interventions on motor control and recovery post-stroke. In the examined studies the pre- and post-treatment analysis showed a significant improvement in almost all clinical outcomes, even when no significant differences in muscle synergies numbers were observed. Hesam Shariati et al. in their muscle synergies analysis, explored difference in level of impairment in stroke patients, demonstrating that synergy characteristics may differ according to the level of motor-function.63 They found a non-significant changes in number only for low to moderate levels of motor function. According to Maistrello et al., the reason for this finding could be a different sensitivity to the recovery level after stroke.58 They argued that the number and merging of muscles synergies alone are not adequate indicators of treatment effectiveness.58 For the authors, the number of muscle synergies may reflect motor recovery, which has been shown to have a positive correlation with the number of synergies of affected limbs and a negative correlation with motor ability.
However, the adaptability and efficiency of muscle synergies, particularly in response to rehabilitation treatment were found across these studies. Alnajjar et al. observed that there was notable modulation in muscle synergies after a rehabilitative training, mirroring healthy individuals adapting to new or unpredictable conditions.59 This congruence in muscle synergy adaptation suggests a foundational mechanism through which the CNS optimizes upper limb movement by tuning possible motor solutions, similar to what happens in healthy participants dealing with unfamiliar environments. Furthermore, initial movements in these unfamiliar environments, while energy-inefficient, serve as prompt reactions. But with training, energy expenditure decreases as more optimal motor solutions are identified.59 This hypothesis was further corroborated by Seo et al., who demonstrated that, after a six-week training period, only the healthy participants who were trained in an unfamiliar task exhibited changes in muscle synergy composition.125
In the clinical context, these findings could significantly influence the development of more effective and energy-efficient rehabilitative training protocols, particularly combining muscle synergies analysis. Maistrello et al. examined the Median-sp was closely related to the patients’ motor outcomes both before and after the treatment. They suggested that Median-sp is a reliable indicator of motor performance following motor treatment.58 In Seo et al., the change in disparity index was significantly correlated with the changed FMA and with the change in reaching distance across all subjects.64 This suggests that reducing abnormal co-activation of trained muscle pairs was associated with an increased range of motion after stroke and with improved motor function after stroke. These findings mirror the broader literature, with some studies associating a decrease in the number of muscle synergies with impaired motor function,114 similar to the motor control patterns of healthy individuals.35
Future works and limitations
The reviewed studies underscore the broad adaptability of muscle synergy analysis in various rehabilitation settings, suggesting its potential to refine both traditional and robot-assisted treatments. The evolving field of stroke rehabilitation calls for deeper insight into recovery mechanisms and the efficacy of interventions, including robotics. According to a recent Consensus conference, future perspectives for robot-assisted rehabilitation in people with neurological conditions include the need to design research studies aimed at investigating the role of robotic and electromechanical devices in promoting neuroplasticity.45 Moreover, for the authors, it is important to develop and apply new theoretical models to better understand the underlying mechanisms of robot-assisted rehabilitation and improve treatment effectiveness.45
The analysis of muscle synergies, for instance, offers a window into our comprehension of recovery dynamics but also paves the way for more targeted and effective therapeutic interventions in the future. However, integrating and exploiting muscle synergies into clinical practice is a process with several challenges. Real-time acquisition and interpretation of muscle synergies data requires specialized expertise, challenging professionals without extensive training. Furthermore, the need for specific, often costly equipment may limit its accessibility in many clinical environments. Lastly, while multidomain assessments offer comprehensive insights, they add complexity to the rehabilitation process, potentially making it challenging for professionals to integrate and act upon such multifaceted data in clinical scenarios.
The reviewed studies used muscle synergies solely to gauge post-rehabilitation effects. It’s worth noting that muscle synergies may provide a more accurate representation of neural damage than motor scales, which may have lower sensitivity.6 This characteristic should be considered when designing and adjusting rehabilitation protocols according to changes in muscle synergies, by creating rehabilitation approaches based on a synergistic rationale. Additionally, rehabilitation protocols and synergy protocols may need to be adjusted over time as the individual progresses. The use of machine learning and synergy-based protocol may improve the personalization of the rehabilitation treatments. Four reviewed studies proposed a synergy-based protocol of treatment.57, 61, 62, 68 Among these, Zendehbad et al. used machine learning to design a nonlinear model based on an artificial neural network to predict the desired upper limb movement trajectory.61 Moreover, the integration of sensors, such as sEMG, with robotics is also expected to bring to the development of more advanced rehabilitation devices that can provide patients with different levels of assistance and support during therapy sessions.126 This represents a promising future direction for muscle synergy-based rehabilitation approaches in stroke. Indeed, the use of sEMG for muscle synergies is still to be explored. For example, some researchers used High definition sEMG suggesting that the modular (synergistic) organization of movement is not at the muscle level but rather at the motor unit level,127 and these two levels do not overlap. This means that rehabilitation protocols should focus on the specific motor units that are responsible for generating the desired movement, rather than simply targeting the muscle as a whole.127 This could yield more efficient training and deeper insights into neural mechanisms.
One possible application is the use of muscle synergies in Human-Robot Interaction, a field that studies how humans and robots interact with each other. For instance, several studies have investigated hand muscle synergies for robotic hand control for rehabilitation purposes.128, 129 Nevertheless, in our research only one study has examined the effects of a hand robot on stroke patients using muscle synergy analysis.53 A possible reason for this could be the challenges involved in measuring the activity of forearm and hand muscles using sEMG.93
The scoping review outlines key challenges in current muscle synergies research in stroke rehabilitation. A significant limitation stems from the heterogeneity in methodologies used for synergy extraction, particularly the diverse algorithms chosen, which hinders direct comparisons between studies. Additionally, the multitude of rehabilitation protocols observed introduces variability in outcomes, complicating the task of establishing standardized intervention guidelines based on muscle synergy analyses. Compounding these challenges is the broad spectrum of patient demographics and intervention groups across the studies. Such diversity underscores the pressing need for more uniform and standardized research protocols to ensure consistent findings.
In summary our scoping review identifies several areas needing further exploration in future longitudinal studies. The choice of synergy protocols significantly impacts the validity and reliability of muscle synergy analysis. For instance, fixed-number synergy protocols may miss individual variability and recovery patterns. Similarly, single-task synergy protocols may not capture the functional diversity of rehabilitation protocols or the range of available synergies and do not allow to recover the repertoire of available muscle synergies. Therefore, choosing a synergy protocol compatible with the rehabilitation protocol is crucial for meaningful, accurate muscle synergy analysis. On the other hand, it is important to evaluate transferability of functional tasks in muscle synergies. Table I provides a succinct overview of these identified areas along with corresponding suggestions, offering a roadmap for researchers and clinicians to enhance the effectiveness and precision of future studies in this domain.
Table I. —List of suggestions for future longitudinal studies based on muscle synergies.
| Aspect | Suggestions |
|---|---|
| Enrolled patients and volunteers | Detailed characterization of the study population. Larger sample size. Patient-specific analyses to minimize inter-subject variability. Careful selection of control groups for accurate comparisons. Uniform definition of stroke type and recovery stage. |
| Types of interventions | Optimization of robotic devices and protocols for effectiveness. Investigation of different forms of robotic assistance. Exploration of muscle synergies across various rehabilitative contexts. |
| Protocols for rehabilitation sessions | Incorporation of movement variability in protocols. Comparison between patient’s affected and unaffected limb and healthy subject’s limb. Introduction of complex, 3D and multi-planar movements. Personalized rehabilitation programs based on muscle synergies. |
| Protocols for synergy extraction | Use of diverse models and algorithms for synergy extraction. Consideration of temporal coefficients in synergy analyses. Standardization of electrode positioning. Training and familiarization of subjects with experimental tasks. |
| Variation of the number of synergies | Investigation of the impact of interventions on muscle synergy number. Understanding of the relationship between synergy number and motor impairment severity. When possible, avoid fixing the number of synergies a priori. Use multiple R2/VAF thresholds or more accurate methods for determining the number of muscle synergies. |
| Variation of synergies structure | Examination of how interventions impact muscle synergy structure. Understanding of the relationship between muscle synergy structure and motor function recovery. |
| Variation of temporal coefficients | Emphasis on changes in temporal coefficients post-intervention. Exploration of the relationship between temporal coefficients and motor function recovery. |
| Clinical and instrumental findings | Investigation of the relationship between muscle synergies and clinical outcomes. Use of multidomain assessments (e.g., kinematics, EEG). Exploration of muscle synergies as a biomarker for motor impairment and recovery. |
Despite thorough efforts to ensure a comprehensive and unbiased review, our methodology entailed certain limitations. Firstly, the inclusion criteria were not restricted solely RCTs, which potentially introduced variability in study designs and heterogeneity in results. Additionally, the research did not encompass all existing databases. Despite these limitations, efforts were made to mitigate bias and ensure a broad coverage of the literature, to provide a holistic view of the topic under review.
Conclusions
This scoping review paper has summarized the current state of knowledge on the effects of neurorehabilitation on muscle synergies in post-stroke patients. Muscle synergies are hypothesized to reflect the modular organization of motor control in the CNS, and their alterations after stroke may indicate impaired motor function and recovery. Furthermore, while robotic treatments have shown promising results in improving muscle synergies, they are still in the early stages of development and require further research to determine their effectiveness in clinical settings. These findings suggest that muscle synergy analysis can provide valuable information on the neural mechanisms underlying motor recovery after stroke, and that it can be used as a biomarker to evaluate the effectiveness of different rehabilitation interventions. However, more studies are needed to establish the causal relationship between muscle synergy changes and functional outcomes, to identify the optimal parameters and protocols for muscle synergy extraction and comparison, and to explore the potential of muscle synergy-based feedback and stimulation for enhancing neuroplasticity and motor learning. In summary, muscle synergy analysis is a promising tool for clinical evaluation and rehabilitation of movement in post-stroke patients.
Supplementary Digital Material 1
Supplementary Table I
Main information of the studies selected in this scoping review: study characteristics and clinical results.
Footnotes
Conflicts of interest: The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding: The authors report no involvement in the research by the sponsor that could have influenced the outcome of this work. This work was supported in part by the Italian Ministry of Research within the project “Fit4MedRob—Fit for Medical Robotics”, Piano Nazionale Complementare (PNC)—PNC0000007.
References
- 1.Feigin VL, Brainin M, Norrving B, Martins S, Sacco RL, Hacke W, et al. World Stroke Organization (WSO): Global Stroke Fact Sheet 2022. Int J Stroke 2022;17:18–29. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34986727&dopt=Abstract 10.1177/17474930211065917 [DOI] [PubMed] [Google Scholar]
- 2.Kwakkel G, Kollen BJ. Predicting activities after stroke: what is clinically relevant? Int J Stroke 2013;8:25–32. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23280266&dopt=Abstract 10.1111/j.1747-4949.2012.00967.x [DOI] [PubMed] [Google Scholar]
- 3.Alt Murphy M, Resteghini C, Feys P, Lamers I. An overview of systematic reviews on upper extremity outcome measures after stroke. BMC Neurol 2015;15:29. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25880033&dopt=Abstract 10.1186/s12883-015-0292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Santisteban L, Térémetz M, Bleton JP, Baron JC, Maier MA, Lindberg PG. Upper Limb Outcome Measures Used in Stroke Rehabilitation Studies: A Systematic Literature Review. PLoS One 2016;11:e0154792. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27152853&dopt=Abstract 10.1371/journal.pone.0154792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thompson-Butel AG, Lin G, Shiner CT, McNulty PA. Comparison of three tools to measure improvements in upper-limb function with poststroke therapy. Neurorehabil Neural Repair 2015;29:341–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25209302&dopt=Abstract 10.1177/1545968314547766 [DOI] [PubMed] [Google Scholar]
- 6.Demers M, Levin MF. Do Activity Level Outcome Measures Commonly Used in Neurological Practice Assess Upper-Limb Movement Quality. Neurorehabil Neural Repair 2017;31:623–37. 10.1177/1545968317714576 [DOI] [PubMed] [Google Scholar]
- 7.Roh J, Rymer WZ, Perreault EJ, Yoo SB, Beer RF. Alterations in upper limb muscle synergy structure in chronic stroke survivors. J Neurophysiol 2013;109:768–81. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23155178&dopt=Abstract 10.1152/jn.00670.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pan B, Sun Y, Xie B, Huang Z, Wu J, Hou J, et al. Alterations of muscle synergies during voluntary arm reaching movement in subacute stroke survivors at different levels of impairment. Front Comput Neurosci 2018;12:69. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30186130&dopt=Abstract 10.3389/fncom.2018.00069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brunnstrom S. Movement Therapy in Hemiplegia: A Neurophysiological Approach. Gerontologist 1970;1. [Google Scholar]
- 10.McMorland AJ, Runnalls KD, Byblow WD. A neuroanatomical framework for upper limb synergies after stroke. Front Hum Neurosci 2015;9:82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25762917&dopt=Abstract 10.3389/fnhum.2015.00082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kwakkel G, Kollen BJ, van der Grond J, Prevo AJ. Probability of regaining dexterity in the flaccid upper limb: impact of severity of paresis and time since onset in acute stroke. Stroke 2003;34:2181–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12907818&dopt=Abstract 10.1161/01.STR.0000087172.16305.CD [DOI] [PubMed] [Google Scholar]
- 12.Banks CL, Pai MM, McGuirk TE, Fregly BJ, Patten C. Methodological Choices in Muscle Synergy Analysis Impact Differentiation of Physiological Characteristics Following Stroke. Front Comput Neurosci 2017;11:78. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28912707&dopt=Abstract 10.3389/fncom.2017.00078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.d’Avella A, Saltiel P, Bizzi E. Combinations of muscle synergies in the construction of a natural motor behavior. Nat Neurosci 2003;6:300–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12563264&dopt=Abstract 10.1038/nn1010 [DOI] [PubMed] [Google Scholar]
- 14.Bizzi E, Cheung VC, d’Avella A, Saltiel P, Tresch M. Combining modules for movement. Brain Res Brain Res Rev 2008;57:125–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18029291&dopt=Abstract 10.1016/j.brainresrev.2007.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharif Razavian R, Mehrabi N, McPhee J. A model-based approach to predict muscle synergies using optimization: application to feedback control. Front Comput Neurosci 2015;9:121. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26500530&dopt=Abstract 10.3389/fncom.2015.00121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Todorov E, Li W, Pan X. From task parameters to motor synergies: A hierarchical framework for approximately-optimal control of redundant manipulators. J Robot Syst 2005;22:691–710. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17710121&dopt=Abstract 10.1002/rob.20093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brambilla C, Atzori M, Müller H, d’Avella A, Scano A. Spatial and Temporal Muscle Synergies Provide a Dual Characterization of Low-dimensional and Intermittent Control of Upper-limb Movements. Neuroscience 2023;514:100–22. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36708799&dopt=Abstract 10.1016/j.neuroscience.2023.01.017 [DOI] [PubMed] [Google Scholar]
- 18.Tresch MC, Cheung VC, d’Avella A. Matrix factorization algorithms for the identification of muscle synergies: evaluation on simulated and experimental data sets. J Neurophysiol 2006;95:2199–212. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16394079&dopt=Abstract 10.1152/jn.00222.2005 [DOI] [PubMed] [Google Scholar]
- 19.Bizzi E, Cheung VC. The neural origin of muscle synergies. Front Comput Neurosci 2013;7:51. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23641212&dopt=Abstract 10.3389/fncom.2013.00051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Overduin SA, d’Avella A, Roh J, Bizzi E. Modulation of muscle synergy recruitment in primate grasping. J Neurosci 2008;28:880–92. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18216196&dopt=Abstract 10.1523/JNEUROSCI.2869-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.d’Avella A, Portone A, Fernandez L, Lacquaniti F. Control of fast-reaching movements by muscle synergy combinations. J Neurosci 2006;26:7791–810. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16870725&dopt=Abstract 10.1523/JNEUROSCI.0830-06.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dominici N, Ivanenko YP, Cappellini G, d’Avella A, Mondì V, Cicchese M, et al. Locomotor primitives in newborn babies and their development. Science 2011;334:997–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22096202&dopt=Abstract 10.1126/science.1210617 [DOI] [PubMed] [Google Scholar]
- 23.Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci USA 2009;106:19563–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19880747&dopt=Abstract 10.1073/pnas.0910114106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tresch MC, Jarc A. The case for and against muscle synergies. Curr Opin Neurobiol 2009;19:601–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19828310&dopt=Abstract 10.1016/j.conb.2009.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Burkholder TJ, van Antwerp KW. Practical limits on muscle synergy identification by non-negative matrix factorization in systems with mechanical constraints. Med Biol Eng Comput 2013;51:187–96. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23124815&dopt=Abstract 10.1007/s11517-012-0983-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Rugy A, Loeb GE, Carroll TJ. Are muscle synergies useful for neural control? Front Comput Neurosci 2013;7:19. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23519326&dopt=Abstract 10.3389/fncom.2013.00019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kutch JJ, Valero-Cuevas FJ. Challenges and new approaches to proving the existence of muscle synergies of neural origin. PLOS Comput Biol 2012;8:e1002434. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22570602&dopt=Abstract 10.1371/journal.pcbi.1002434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ting LH, McKay JL. Neuromechanics of muscle synergies for posture and movement. Curr Opin Neurobiol 2007;17:622–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18304801&dopt=Abstract 10.1016/j.conb.2008.01.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alessandro C, Delis I, Nori F, Panzeri S, Berret B. Muscle synergies in neuroscience and robotics: from input-space to task-space perspectives. Front Comput Neurosci 2013;7:43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23626535&dopt=Abstract 10.3389/fncom.2013.00043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lee DD, Seung HS. Learning the parts of objects by non-negative matrix factorization. Nature 1999;401:788–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10548103&dopt=Abstract 10.1038/44565 [DOI] [PubMed] [Google Scholar]
- 31.Ranganathan R, Krishnan C. Extracting synergies in gait: using EMG variability to evaluate control strategies. J Neurophysiol 2012;108:1537–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22723678&dopt=Abstract 10.1152/jn.01112.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rasool G, Iqbal K, Bouaynaya N, White G. Real-Time Task Discrimination for Myoelectric Control Employing Task-Specific Muscle Synergies. IEEE Trans Neural Syst Rehabil Eng 2016;24:98–108. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25769166&dopt=Abstract 10.1109/TNSRE.2015.2410176 [DOI] [PubMed] [Google Scholar]
- 33.Clark DJ, Ting LH, Zajac FE, Neptune RR, Kautz SA. Merging of healthy motor modules predicts reduced locomotor performance and muscle coordination complexity post-stroke. J Neurophysiol 2010;103:844–57. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20007501&dopt=Abstract 10.1152/jn.00825.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li S, Zhuang C, Niu CM, Bao Y, Xie Q, Lan N. Evaluation of functional correlation of task-specific muscle synergies with motor performance in patients poststroke. Front Neurol 2017;8:337. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28785238&dopt=Abstract 10.3389/fneur.2017.00337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung VC, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, et al. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci USA 2012;109:14652–6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22908288&dopt=Abstract 10.1073/pnas.1212056109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roh J, Rymer WZ, Beer RF. Evidence for altered upper extremity muscle synergies in chronic stroke survivors with mild and moderate impairment. Front Hum Neurosci 2015;9:6. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25717296&dopt=Abstract 10.3389/fnhum.2015.00006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.García-Cossio E, Broetz D, Birbaumer N, Ramos-Murguialday A. Cortex integrity relevance in muscle synergies in severe chronic stroke. Front Hum Neurosci 2014;8:744. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=25294998&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anwer S, Waris A, Gilani SO, Iqbal J, Shaikh N, Pujari AN, et al. Rehabilitation of Upper Limb Motor Impairment in Stroke: A Narrative Review on the Prevalence, Risk Factors, and Economic Statistics of Stroke and State of the Art Therapies. Healthcare (Basel) 2022;10. 10.3390/healthcare10020190 [DOI] [PMC free article] [PubMed]
- 39.Hatem SM, Saussez G, Della Faille M, Prist V, Zhang X, Dispa D, et al. Rehabilitation of Motor Function after Stroke: A Multiple Systematic Review Focused on Techniques to Stimulate Upper Extremity Recovery. Front Hum Neurosci 2016;10:442. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27679565&dopt=Abstract 10.3389/fnhum.2016.00442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Volpe BT, Krebs HI, Hogan N, Edelstein OTR L, Diels C, Aisen M. A novel approach to stroke rehabilitation: robot-aided sensorimotor stimulation. Neurology 2000;54:1938–44. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10822433&dopt=Abstract 10.1212/WNL.54.10.1938 [DOI] [PubMed] [Google Scholar]
- 41.Blank AA, French JA, Pehlivan AU, O’Malley MK. Current Trends in Robot-Assisted Upper-Limb Stroke Rehabilitation: Promoting Patient Engagement in Therapy. Curr Phys Med Rehabil Rep 2014;2:184–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26005600&dopt=Abstract 10.1007/s40141-014-0056-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pournajaf S, Morone G, Straudi S, Goffredo M, Leo MR, Calabrò RS, et al. The Italian PowerUPS-Rehab Study Group . Neurophysiological and Clinical Effects of Upper Limb Robot-Assisted Rehabilitation on Motor Recovery in Patients with Subacute Stroke: A Multicenter Randomized Controlled Trial Study Protocol. Brain Sci 2023;13:700. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37190665&dopt=Abstract 10.3390/brainsci13040700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panarese A, Colombo R, Sterpi I, Pisano F, Micera S. Tracking motor improvement at the subtask level during robot-aided neurorehabilitation of stroke patients. Neurorehabil Neural Repair 2012;26:822–33. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22374174&dopt=Abstract 10.1177/1545968311431966 [DOI] [PubMed] [Google Scholar]
- 44.Clark WE, Sivan M, O’Connor RJ. Evaluating the use of robotic and virtual reality rehabilitation technologies to improve function in stroke survivors: A narrative review. J Rehabil Assist Technol Eng 2019;6:2055668319863557. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31763052&dopt=Abstract 10.1177/2055668319863557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Turolla A, Kiper P, Mazzarotto D, Cecchi F, Colucci M, D’Avenio G, et al. Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE) . Reference theories and future perspectives on robot-assisted rehabilitation in people with neurological conditions: A scoping review and recommendations from the Italian Consensus Conference on Robotics in Neurorehabilitation (CICERONE). NeuroRehabilitation 2022;51:681–91. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36530100&dopt=Abstract 10.3233/NRE-220160 [DOI] [PubMed] [Google Scholar]
- 46.Rodgers H, Bosomworth H, Krebs HI, van Wijck F, Howel D, Wilson N, et al. Robot assisted training for the upper limb after stroke (RATULS): a multicentre randomised controlled trial. Lancet 2019;394:51–62. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31128926&dopt=Abstract 10.1016/S0140-6736(19)31055-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong YN, Ballekere AN, Fregly BJ, Roh J. Are muscle synergies useful for stroke rehabilitation? Curr Opin Biomed Eng 2021;19:100315. 10.1016/j.cobme.2021.100315 [DOI] [Google Scholar]
- 48.Seamon BA, Neptune RR, Kautz SA. Using a Module-Based Analysis Framework for Investigating Muscle Coordination during Walking in Individuals Poststroke: A Literature Review and Synthesis. Appl Bionics Biomech 2018;2018:3795754. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=29967653&dopt=Abstract 10.1155/2018/3795754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Peters MD, Marnie C, Tricco AC, Pollock D, Munn Z, Alexander L, et al. Updated methodological guidance for the conduct of scoping reviews. JBI Evid Synth 2020;18:2119–26. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33038124&dopt=Abstract 10.11124/JBIES-20-00167 [DOI] [PubMed] [Google Scholar]
- 50.Tricco AC, Lillie E, Zarin W, O’Brien KK, Colquhoun H, Levac D, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med 2018;169:467–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30178033&dopt=Abstract 10.7326/M18-0850 [DOI] [PubMed] [Google Scholar]
- 51.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021;372:n71. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33782057&dopt=Abstract 10.1136/bmj.n71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pierella C, Pirondini E, Kinany N, Coscia M, Giang C, Miehlbradt J, et al. A multimodal approach to capture post-stroke temporal dynamics of recovery. J Neural Eng 2020;17:045002. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32516757&dopt=Abstract 10.1088/1741-2552/ab9ada [DOI] [PubMed] [Google Scholar]
- 53.Di Luzio FS, Cordella F, Bravi M, Santacaterina F, Bressi F, Sterzi S, et al. Modification of Hand Muscular Synergies in Stroke Patients after Robot-Aided Rehabilitation. Appl Sci (Basel) 2022;12:3146. 10.3390/app12063146 [DOI] [Google Scholar]
- 54.Belfatto A, Scano A, Chiavenna A, Mastropietro A, Mrakic-Sposta S, Pittaccio S, et al. A Multiparameter Approach to Evaluate Post-Stroke Patients: An Application on Robotic Rehabilitation. Appl Sci (Basel) 2018;8:2248. 10.3390/app8112248 [DOI] [Google Scholar]
- 55.Tropea P, Monaco V, Coscia M, Posteraro F, Micera S. Effects of early and intensive neuro-rehabilitative treatment on muscle synergies in acute post-stroke patients: a pilot study. J Neuroeng Rehabil 2013;10:103. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24093623&dopt=Abstract 10.1186/1743-0003-10-103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Irastorza-Landa N, García-Cossio E, Sarasola-Sanz A, Brötz D, Birbaumer N, Ramos-Murguialday A. Functional synergy recruitment index as a reliable biomarker of motor function and recovery in chronic stroke patients. J Neural Eng 2021;18:046061. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33530072&dopt=Abstract 10.1088/1741-2552/abe244 [DOI] [PubMed] [Google Scholar]
- 57.Niu CM, Bao Y, Zhuang C, Li S, Wang T, Cui L, et al. Synergy-Based FES for Post-Stroke Rehabilitation of Upper-Limb Motor Functions. IEEE Trans Neural Syst Rehabil Eng 2019;27:256–64. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30763238&dopt=Abstract 10.1109/TNSRE.2019.2891004 [DOI] [PubMed] [Google Scholar]
- 58.Maistrello L, Rimini D, Cheung VC, Pregnolato G, Turolla A. Muscle Synergies and Clinical Outcome Measures Describe Different Factors of Upper Limb Motor Function in Stroke Survivors Undergoing Rehabilitation in a Virtual Reality Environment. Sensors (Basel) 2021;21:8002. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34884003&dopt=Abstract 10.3390/s21238002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alnajjar FS, Moreno JC, Ozaki KI, Kondo I, Shimoda S. Motor Control System for Adaptation of Healthy Individuals and Recovery of Poststroke Patients: A Case Study on Muscle Synergies. Neural Plast 2019;2019:8586416. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31049057&dopt=Abstract 10.1155/2019/8586416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lencioni T, Fornia L, Bowman T, Marzegan A, Caronni A, Turolla A, et al. A randomized controlled trial on the effects induced by robot-assisted and usual-care rehabilitation on upper limb muscle synergies in post-stroke subjects. Sci Rep 2021;11:5323. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=33674675&dopt=Abstract 10.1038/s41598-021-84536-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zendehbad SA, Kobravi HR, Khalilzadeh MM, Razavi AS, Nezhad PS. Presenting a New Muscle Synergy Analysis based Mechanism to Design a Trackable Visual Biofeedback Signal: Applicable to Arm Movement Recovery after Ischemic Stroke. IEEE Access 2023. 10.1109/ACCESS.2023.3287408 [DOI]
- 62.Niu CM, Chou CH, Bao Y, Wang T, Gu L, Zhang X, et al. A pilot study of synergy-based FES for upper-extremity poststroke rehabilitation. Neurosci Lett 2022;780:136621. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35395324&dopt=Abstract 10.1016/j.neulet.2022.136621 [DOI] [PubMed] [Google Scholar]
- 63.Hesam-Shariati N, Trinh T, Thompson-Butel AG, Shiner CT, McNulty PA. A longitudinal electromyography study of complex movements in poststroke therapy. 2: changes in coordinated muscle activation. Front Neurol 2017;8:277. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28775705&dopt=Abstract 10.3389/fneur.2017.00277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Seo G, Kishta A, Mugler E, Slutzky MW, Roh J. Myoelectric interface training enables targeted reduction in abnormal muscle co-activation. J Neuroeng Rehabil 2022;19:67. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35778757&dopt=Abstract 10.1186/s12984-022-01045-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hesam-Shariati N, Trinh T, Thompson-Butel AG, Shiner CT, McNulty PA. A Longitudinal Electromyography Study of Complex Movements in Poststroke Therapy. 1: Heterogeneous Changes Despite Consistent Improvements in Clinical Assessments. Front Neurol 2017;8:340. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28804474&dopt=Abstract 10.3389/fneur.2017.00340 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mugler EM, Tomic G, Singh A, Hameed S, Lindberg EW, Gaide J, et al. Myoelectric Computer Interface Training for Reducing Co-Activation and Enhancing Arm Movement in Chronic Stroke Survivors: A Randomized Trial. Neurorehabil Neural Repair 2019;33:284–95. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30888251&dopt=Abstract 10.1177/1545968319834903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fugl-Meyer AR, Jääskö L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand J Rehabil Med 1975;7:13–31. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1135616&dopt=Abstract 10.2340/1650197771331 [DOI] [PubMed] [Google Scholar]
- 68.Dash A, Lahiri U. Design of Virtual Reality-Enabled Surface Electromyogram-Triggered Grip Exercise Platform. IEEE Trans Neural Syst Rehabil Eng 2020;28:444–52. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31841415&dopt=Abstract 10.1109/TNSRE.2019.2959449 [DOI] [PubMed] [Google Scholar]
- 69.Maciejasz P, Eschweiler J, Gerlach-Hahn K, Jansen-Troy A, Leonhardt S. A survey on robotic devices for upper limb rehabilitation. J Neuroeng Rehabil 2014;11:3. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24401110&dopt=Abstract 10.1186/1743-0003-11-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Mehrholz J, Pohl M, Platz T, Kugler J, Elsner B. Electromechanical and robot-assisted arm training for improving activities of daily living, arm function, and arm muscle strength after stroke. Cochrane Database Syst Rev 2018;9:CD006876. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30175845&dopt=Abstract 10.1002/14651858.CD006876.pub5 [DOI] [PMC free article] [PubMed]
- 71.Hayward KS, Kramer SF, Dalton EJ, Hughes GR, Brodtmann A, Churilov L, et al. Timing and Dose of Upper Limb Motor Intervention After Stroke: A Systematic Review. Stroke 2021;52:3706–17. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34601901&dopt=Abstract 10.1161/STROKEAHA.121.034348 [DOI] [PubMed] [Google Scholar]
- 72.Bernhardt J, Hayward KS, Kwakkel G, Ward NS, Wolf SL, Borschmann K, et al. Agreed Definitions and a Shared Vision for New Standards in Stroke Recovery Research: The Stroke Recovery and Rehabilitation Roundtable Taskforce. Neurorehabil Neural Repair 2017;31:793–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28934920&dopt=Abstract 10.1177/1545968317732668 [DOI] [PubMed] [Google Scholar]
- 73.Langhorne P, Bernhardt J, Kwakkel G. Stroke rehabilitation. Lancet 2011;377:1693–702. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21571152&dopt=Abstract 10.1016/S0140-6736(11)60325-5 [DOI] [PubMed] [Google Scholar]
- 74.Dobkin BH. Strategies for stroke rehabilitation. Lancet Neurol 2004;3:528–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15324721&dopt=Abstract 10.1016/S1474-4422(04)00851-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bustrén EL, Sunnerhagen KS, Alt Murphy M. Movement Kinematics of the Ipsilesional Upper Extremity in Persons With Moderate or Mild Stroke. Neurorehabil Neural Repair 2017;31:376–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28107802&dopt=Abstract 10.1177/1545968316688798 [DOI] [PubMed] [Google Scholar]
- 76.Morasso P, Casadio M, Giannoni P, Masia L, Sanguineti V, Squeri V, et al. Desirable features of a “humanoid” robot-therapist. Annu Int Conf IEEE Eng Med Biol Soc 2009;2009:2418–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=19965200&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 77.Krakauer JW. Motor learning: its relevance to stroke recovery and neurorehabilitation. Curr Opin Neurol 2006;19:84–90. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16415682&dopt=Abstract 10.1097/01.wco.0000200544.29915.cc [DOI] [PubMed] [Google Scholar]
- 78.Shmuelof L, Krakauer JW, Mazzoni P. How is a motor skill learned? Change and invariance at the levels of task success and trajectory control. J Neurophysiol 2012;108:578–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22514286&dopt=Abstract 10.1152/jn.00856.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Salmoni AW, Schmidt RA, Walter CB. Knowledge of results and motor learning: a review and critical reappraisal. Psychol Bull 1984;95:355–86. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6399752&dopt=Abstract 10.1037/0033-2909.95.3.355 [DOI] [PubMed] [Google Scholar]
- 80.Winstein CJ. Knowledge of results and motor learning—implications for physical therapy. Phys Ther 1991;71:140–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1989009&dopt=Abstract 10.1093/ptj/71.2.140 [DOI] [PubMed] [Google Scholar]
- 81.Milot MH, Spencer SJ, Chan V, Allington JP, Klein J, Chou C, et al. A crossover pilot study evaluating the functional outcomes of two different types of robotic movement training in chronic stroke survivors using the arm exoskeleton BONES. J Neuroeng Rehabil 2013;10:112. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24354476&dopt=Abstract 10.1186/1743-0003-10-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Marchal-Crespo L, Schneider J, Jaeger L, Riener R. Learning a locomotor task: with or without errors? J Neuroeng Rehabil 2014;11:25. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24594267&dopt=Abstract 10.1186/1743-0003-11-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cancrini A, Baitelli P, Lavit Nicora M, Malosio M, Pedrocchi A, Scano A. The effects of robotic assistance on upper limb spatial muscle synergies in healthy people during planar upper-limb training. PLoS One 2022;17:e0272813. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35939495&dopt=Abstract 10.1371/journal.pone.0272813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jarrassé N, Paik J, Pasqui V, Morel G. How can human motion prediction increase transparency? Proc IEEE Int Conf Robot Autom. 2008;2134–9. [Google Scholar]
- 85.Duret C, Grosmaire AG, Krebs HI. Robot-Assisted Therapy in Upper Extremity Hemiparesis: Overview of an Evidence-Based Approach. Front Neurol 2019;10:412. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31068898&dopt=Abstract 10.3389/fneur.2019.00412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kwakkel G, Kollen BJ, Krebs HI. Effects of robot-assisted therapy on upper limb recovery after stroke: a systematic review. Neurorehabil Neural Repair 2008;22:111–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17876068&dopt=Abstract 10.1177/1545968307305457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Scano A, Dardari L, Molteni F, Giberti H, Tosatti LM, d’Avella A. A comprehensive spatial mapping of muscle synergies in highly variable upper-limb movements of healthy subjects. Front Physiol 2019;10:1231. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=31611812&dopt=Abstract 10.3389/fphys.2019.01231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhao K, Zhang Z, Wen H, Liu B, Li J, Andrea d’Avella, et al. Muscle synergies for evaluating upper limb in clinical applications: A systematic review. Heliyon 2023;9:e16202. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37215841&dopt=Abstract 10.1016/j.heliyon.2023.e16202 [DOI] [PMC free article] [PubMed]
- 89.Stergiou N, Harbourne R, Cavanaugh J. Optimal movement variability: a new theoretical perspective for neurologic physical therapy. J Neurol Phys Ther 2006;30:120–9. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17029655&dopt=Abstract 10.1097/01.NPT.0000281949.48193.d9 [DOI] [PubMed] [Google Scholar]
- 90.Kutsuzawa K, Hayashibe M. Motor synergy generalization framework for new targets in multi-planar and multi-directional reaching task. R Soc Open Sci 2022;9:211721. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35620009&dopt=Abstract 10.1098/rsos.211721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Wu HG, Miyamoto YR, Gonzalez Castro LN, Ölveczky BP, Smith MA. Temporal structure of motor variability is dynamically regulated and predicts motor learning ability. Nat Neurosci 2014;17:312–21. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24413700&dopt=Abstract 10.1038/nn.3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Asai T, Sugimori E, Tanno Y. Two agents in the brain: motor control of unimanual and bimanual reaching movements. PLoS One 2010;5:e10086. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20386749&dopt=Abstract 10.1371/journal.pone.0010086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pale U, Atzori M, Müller H, Scano A. Variability of Muscle Synergies in Hand Grasps: Analysis of Intra- and Inter-Session Data. Sensors (Basel) 2020;20:1–27. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32752155&dopt=Abstract 10.3390/s20154297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Thacham Poyil A, Steuber V, Amirabdollahian F. Adaptive robot mediated upper limb training using electromyogram-based muscle fatigue indicators. PLoS One 2020;15:e0233545. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=32469912&dopt=Abstract 10.1371/journal.pone.0233545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ivanenko YP, Poppele RE, Lacquaniti F. Five basic muscle activation patterns account for muscle activity during human locomotion. J Physiol 2004;556:267–82. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14724214&dopt=Abstract 10.1113/jphysiol.2003.057174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Scano A, Mira RM, d’Avella A. Mixed matrix factorization: a novel algorithm for the extraction of kinematic-muscular synergies. J Neurophysiol 2022;127:529–47. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34986023&dopt=Abstract 10.1152/jn.00379.2021 [DOI] [PubMed] [Google Scholar]
- 97.Delis I, Panzeri S, Pozzo T, Berret B. A unifying model of concurrent spatial and temporal modularity in muscle activity. J Neurophysiol 2014;111:675–93. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24089400&dopt=Abstract 10.1152/jn.00245.2013 [DOI] [PubMed] [Google Scholar]
- 98.Spüler M, Irastorza-Landa N, Sarasola-Sanz A, Ramos-Murguialday A. Extracting muscle synergy patterns from EMG data using autoencoders. Lecture Notes in Computer Science (including subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics). J Neural Eng 2016;988747–54. [Google Scholar]
- 99.Ó’ Reilly D, Delis I. A network information theoretic framework to characterise muscle synergies in space and time. J Neural Eng 2022;19:016031. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35108699&dopt=Abstract 10.1088/1741-2552/ac5150 [DOI] [PubMed] [Google Scholar]
- 100.Cheung VC, d’Avella A, Tresch MC, Bizzi E. Central and sensory contributions to the activation and organization of muscle synergies during natural motor behaviors. J Neurosci 2005;25:6419–34. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16000633&dopt=Abstract 10.1523/JNEUROSCI.4904-04.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mussa-Ivaldi FA, Giszter SF, Bizzi E. Linear combinations of primitives in vertebrate motor control. Proc Natl Acad Sci USA 1994;91:7534–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8052615&dopt=Abstract 10.1073/pnas.91.16.7534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Saltiel P, Wyler-Duda K, D’Avella A, Tresch MC, Bizzi E. Muscle synergies encoded within the spinal cord: evidence from focal intraspinal NMDA iontophoresis in the frog. J Neurophysiol 2001;85:605–19. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11160497&dopt=Abstract 10.1152/jn.2001.85.2.605 [DOI] [PubMed] [Google Scholar]
- 103.Tresch MC, Saltiel P, Bizzi E. The construction of movement by the spinal cord. Nat Neurosci 1999;2:162–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10195201&dopt=Abstract 10.1038/5721 [DOI] [PubMed] [Google Scholar]
- 104.Hart CB, Giszter SF. A neural basis for motor primitives in the spinal cord. J Neurosci 2010;30:1322–36. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20107059&dopt=Abstract 10.1523/JNEUROSCI.5894-08.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Takei T, Confais J, Tomatsu S, Oya T, Seki K. Neural basis for hand muscle synergies in the primate spinal cord. Proc Natl Acad Sci USA 2017;114:8643–8. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28739958&dopt=Abstract 10.1073/pnas.1704328114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.York G, Osborne H, Sriya P, Astill S, de Kamps M, Chakrabarty S. The effect of limb position on a static knee extension task can be explained with a simple spinal cord circuit model. J Neurophysiol 2022;127:173–87. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34879209&dopt=Abstract 10.1152/jn.00208.2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Overduin SA, d’Avella A, Carmena JM, Bizzi E. Microstimulation activates a handful of muscle synergies. Neuron 2012;76:1071–7. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23259944&dopt=Abstract 10.1016/j.neuron.2012.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Drew T, Kalaska J, Krouchev N. Muscle synergies during locomotion in the cat: a model for motor cortex control. J Physiol 2008;586:1239–45. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=18202098&dopt=Abstract 10.1113/jphysiol.2007.146605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Torres-Oviedo G, Macpherson JM, Ting LH. Muscle synergy organization is robust across a variety of postural perturbations. J Neurophysiol 2006;96:1530–46. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16775203&dopt=Abstract 10.1152/jn.00810.2005 [DOI] [PubMed] [Google Scholar]
- 110.Borzelli D, Berger DJ, Pai DK, d’Avella A. Effort minimization and synergistic muscle recruitment for three-dimensional force generation. Front Comput Neurosci 2013;7:186. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24391581&dopt=Abstract 10.3389/fncom.2013.00186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Devarajan K, Cheung VC. On nonnegative matrix factorization algorithms for signal-dependent noise with application to electromyography data. Neural Comput 2014;26:1128–68. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=24684448&dopt=Abstract 10.1162/NECO_a_00576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Steele KM, Tresch MC, Perreault EJ. The number and choice of muscles impact the results of muscle synergy analyses. Front Comput Neurosci 2013;7:105. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23964232&dopt=Abstract 10.3389/fncom.2013.00105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Brambilla C, Scano A. The Number and Structure of Muscle Synergies Depend on the Number of Recorded Muscles: A Pilot Simulation Study with OpenSim. Sensors (Basel) 2022;22:8584. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36433182&dopt=Abstract 10.3390/s22228584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hashiguchi Y, Ohata K, Kitatani R, Yamakami N, Sakuma K, Osako S, et al. Merging and Fractionation of Muscle Synergy Indicate the Recovery Process in Patients with Hemiplegia: The First Study of Patients after Subacute Stroke. Neural Plast 2016;2016:5282957. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=28090358&dopt=Abstract 10.1155/2016/5282957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Scano A, Chiavenna A, Malosio M, Molinari Tosatti L, Molteni F. Robotic Assistance for Upper Limbs May Induce Slight Changes in Motor Modules Compared With Free Movements in Stroke Survivors: A Cluster-Based Muscle Synergy Analysis. Front Hum Neurosci 2018;12:290. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30174596&dopt=Abstract 10.3389/fnhum.2018.00290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Scano A, Guanziroli E, Brambilla C, Amendola C, Pirovano I, Gasperini G, et al. A Narrative Review on Multi-Domain Instrumental Approaches to Evaluate Neuromotor Function in Rehabilitation. Healthcare (Basel) 2023;11:2282. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37628480&dopt=Abstract 10.3390/healthcare11162282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Scano A, Jarque-Bou N, Brambilla C, Atzori M, D’avella A, Muller H. Functional Synergies Applied to a Publicly Available Dataset of Hand Grasps Show Evidence of Kinematic-Muscular Synergistic Control. IEEE Access 2023;11:108544–60. 10.1109/ACCESS.2023.3321510 [DOI]
- 118.Qian C, Li W, Jia T, Li C, Lin PJ, Yang Y, et al. Quantitative Assessment of Motor Function by an End-Effector Upper Limb Rehabilitation Robot Based on Admittance Control. Appl Sci (Basel) 2021;11:6854. 10.3390/app11156854 [DOI] [Google Scholar]
- 119.Cho W, Vidaurre C, An J, Birbaumer N, Ramos-Murguialday A. Cortical processing during robot and functional electrical stimulation. Front Syst Neurosci 2023;17:1045396. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37025164&dopt=Abstract 10.3389/fnsys.2023.1045396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Brambilla C, Pirovano I, Mira RM, Rizzo G, Scano A, Mastropietro A. Combined Use of EMG and EEG Techniques for Neuromotor Assessment in Rehabilitative Applications: A Systematic Review. Sensors (Basel) 2021;21:7014. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34770320&dopt=Abstract 10.3390/s21217014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Vidaurre C, Klauer C, Schauer T, Ramos-Murguialday A, Müller KR. EEG-based BCI for the linear control of an upper-limb neuroprosthesis. Med Eng Phys 2016;38:1195–204. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=27425203&dopt=Abstract 10.1016/j.medengphy.2016.06.010 [DOI] [PubMed] [Google Scholar]
- 122.Terry G, Kayes N. Person centered care in neurorehabilitation: a secondary analysis. Disabil Rehabil 2020;42:2334–43. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=30696295&dopt=Abstract 10.1080/09638288.2018.1561952 [DOI] [PubMed] [Google Scholar]
- 123.Ballarini R, Ghislieri M, Knaflitz M, Agostini V. An Algorithm for Choosing the Optimal Number of Muscle Synergies during Walking. Sensors (Basel) 2021;21:3311. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=34064615&dopt=Abstract 10.3390/s21103311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Funato T, Hattori N, Yozu A, An Q, Oya T, Shirafuji S, et al. Muscle synergy analysis yields an efficient and physiologically relevant method of assessing stroke. Brain Commun 2022;4:fcac200. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=35974798&dopt=Abstract 10.1093/braincomms/fcac200 [DOI] [PMC free article] [PubMed]
- 125.Seo G, Park JH, Park HS, Roh J. Developing new intermuscular coordination patterns through an electromyographic signal-guided training in the upper extremity. J Neuroeng Rehabil 2023;20:112. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37658406&dopt=Abstract 10.1186/s12984-023-01236-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Facciorusso S, Spina S, Reebye R, Turolla A, Calabrò RS, Fiore P, et al. Sensor-Based Rehabilitation in Neurological Diseases: A Bibliometric Analysis of Research Trends. Brain Sci 2023;13:724. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=37239196&dopt=Abstract 10.3390/brainsci13050724 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Del Vecchio A, Marconi Germer C, Kinfe TM, Nuccio S, Hug F, Eskofier B, et al. The Forces Generated by Agonist Muscles during Isometric Contractions Arise from Motor Unit Synergies. J Neurosci 2023;43:2860–73. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=36922028&dopt=Abstract 10.1523/JNEUROSCI.1265-22.2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Grinyagin IV, Biryukova EV, Maier MA. Kinematic and dynamic synergies of human precision-grip movements. J Neurophysiol 2005;94:2284–94. https://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15917316&dopt=Abstract 10.1152/jn.01310.2004 [DOI] [PubMed] [Google Scholar]
- 129.Grioli G, Catalano M, Silvestro E, Tono S, Bicchi A. Adaptive synergies: An approach to the design of under-actuated robotic hands. IEEE International Conference on Intelligent Robots and Systems 2012;1251–6. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I
Main information of the studies selected in this scoping review: study characteristics and clinical results.