Fig. 2|. The enterohepatlc circulation or bile acids.
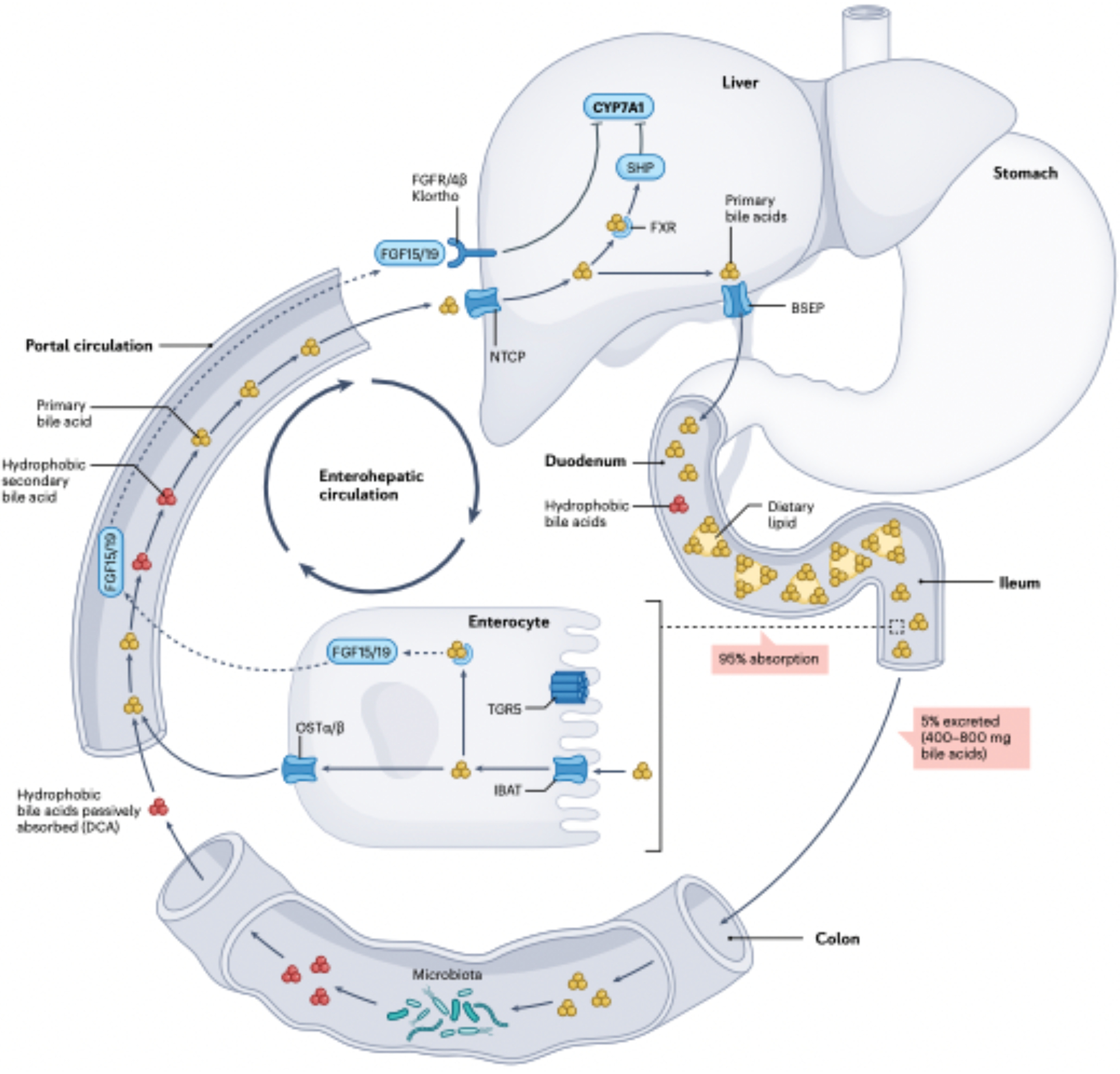
Primary bile acids (yellow) are synthesized de novo in hepatocytes from cholesterol and secreted into bile through transport protein BSEP. During a meal, the gallbladder contracts, releasing bile into the duodenum, where mixed micelles composed of phosphdipods. fatty acids, cholesterol and lipid-soluble vitamins form and are surrounded by amphipathic conjugated bile acids. When conjugated bile acids reach the terminal ileum, they are transposed into enterocytes by the illeal sodium-bile acid cotransporter (JBAT), bound to FABP6 and transported into portal circulation via OSTα and OSTβ expressed basolaterally on enterocytes. As part of the negative feedback function of bile acid synthesis, intracellular bile acids activate the nuclear farnesoild X receptor (FXR) in enterocytes, resulting in upregulation in the synthesis and secretion of the protein FGF15/19 into the portal circulation. FGF15/19 binds to fibroblast growth factor receptor FGFR4/β-Klortho receptor-dependent manner resulting in inhibition of CYP7AL the rate-limiting enzyme in bile acid biosynthesis in the liver. Bile acids returning to the liver are transported by NTCP. Activation of FXR in hepatocytes represses CYP7A1 expression dependent on small heterodimer partner (SHP) and liver-related homologue 1(LRHI). This process allows bile acid levels to remain in steady state. TCRS activation in intestinal stem cells promotes regeneration of enterocytes20. Roughly 5% of bile acids (400–800 mg per day) escape fieal transport and enter the large Intestine, which is the major route by which cholesterol is removed from the body. In the large intestine, bile acid structure and function are diversified by the gut microbiota. Part of this diversification is increasing the hydrophobicity of bile acids in the large intestine, allowing passive absorption into colonocytes and entry into the portal circulation, where secondary bile acids (mainly decoycholic acid (DCA)) accumulate to roughly one-quarter of the bile acid pool in healthy humans.
