Fig. 7.
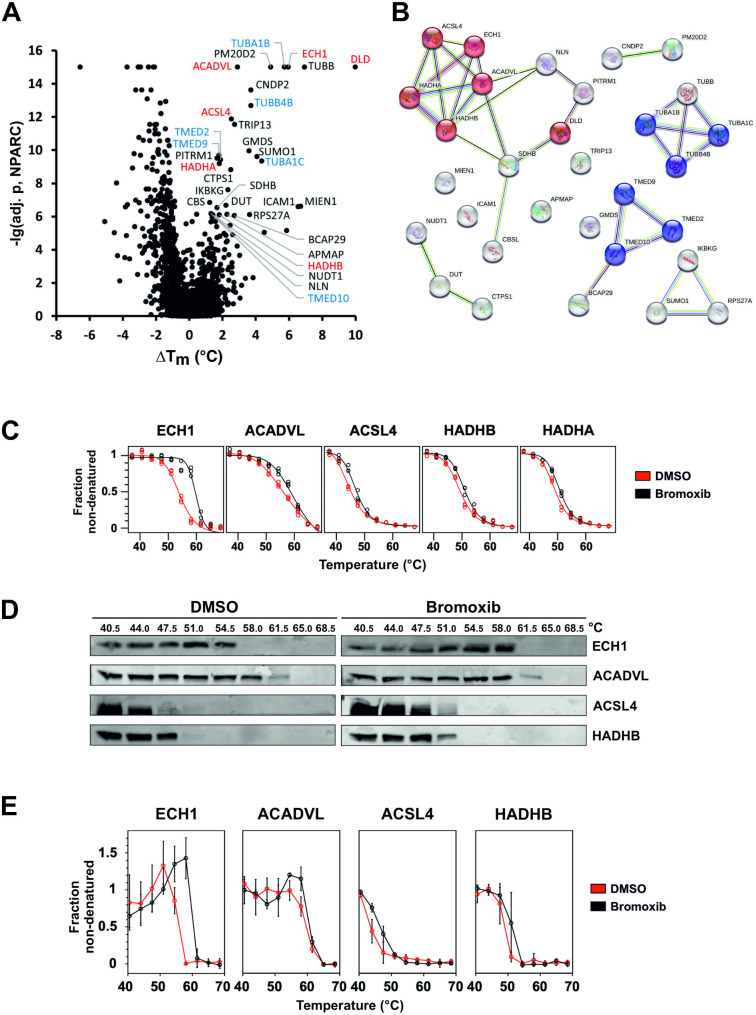
Thermal proteome profiling (TPP) revealed bromoxib-mediated stabilization of multiple proteins. Mass spectrometry based TPP was used to analyze the thermal stabilization and destabilization of proteins upon treatment with bromoxib. For this, Ramos cells were treated with 40 µM bromoxib or a diluent control (DMSO, 0.4% v/v) for a duration of 30 min. (A) Volcano-like plot of the statistical significance (expressed as the negative decadic logarithm of the adjusted NPARC p-value, -lg(adj. p, NPARC)) versus the bromoxib mediated melting point shift (difference of the means of the melting points with and without bromoxib treatment, ΔTm) for each protein. Stabilized proteins (cutoffs: -lg(adj. p, NPARC) > 6 and ΔTm > 0.5°C) are labeled and color coded as in panel B. (B) A functional protein association network (based on a STRING database enrichment analysis, https://string-db.org, v11.5) of the selected 31 stabilized proteins from panel A. Proteins related to ‘fatty acid metabolic process’ (GO:0006631) are shown in red and to ‘COPI-mediated transport’ (‘COPI-mediated anterograde transport’: HSA-6807878 and ‘COPI-dependent Golgi-to-ER retrograde traffic’: HSA-6811434) in blue. The colors of the links indicate the type of interactions (light blue: known interactions from curated databases, purple: known experimentally determined interactions, green: predicted interactions by gene neighborhood, red: predicted interactions by gene fusions, predicted interactions by gene co-occurrence, yellow: text mining, black: co-expression, light purple: protein homology). (C) The five proteins belonging to the ‘fatty acid metabolism’ with their melting curves: (enoyl-CoA hydratase 1 (ECH1), acyl-CoA dehydrogenase very long chain (ACADVL), acyl-CoA synthetase long chain family member 4 (ACSL4), hydroxyacyl-CoA dehydrogenase trifunctional multienzyme complex subunit β (HADHB) and subunit α (HADHA)). Upon bromoxib treatment (40 µM bromoxib for 30 min), a thermal stabilization of these proteins was identified. (D) Exemplary immunoblots (CETSA; see (E) for quantitative analysis) against proteins of the ‘fatty acid metabolism’ (ECH1, ACADVL, ACSL4, and HADHB) employing one of the three independent biological replicates used for the TPP. Since HADHB and HADHA belong to the same protein complex whose subunits have different functions, only HADHB is shown. Upon 40 mM bromoxib treatment for 30 min, thermal stabilization of the proteins can be seen in the immunoblots. (E) Quantitative analysis of immunoblots (CETSA; see (D) for representative examples). The non-denatured fractions of the proteins ECH1, ACADVL, ACSL4, and HADHB from three independent biological replicates (n = 3) were quantified, the normalized signal intensity is shown for DMSO in red and bromoxib in black. Error bars = mean ± SD of three independent biological experiments