Abstract
Background
Intravenous fluid administration and prophylactic vasopressor infusion are the primary methods for preventing spinal anesthesia-induced hypotension during cesarean delivery. However, evidence regarding the impact of different volumes of crystalloid solution on the phenylephrine infusion dosage for preventing this hypotension remains inconclusive. This study aimed to determine the effect of two IV fluid infusion rates (10 or 20 mL/kg/h) on phenylephrine requirement for preventing spinal anesthesia-induced hypotension.
Methods
Eighty healthy parturients undergoing elective cesarean delivery under combined spinal-epidural anesthesia were enrolled. Participants were randomly assigned to receive either 10 mL/kg/h (group 10) or 20 mL/kg/h (group 20) of lactated Ringer’s solution. The first patient in each group received 0.5 µg/kg/min of phenylephrine infusion immediately after intrathecal injection. The phenylephrine dose in subsequent patients was adjusted by increments or decrements of 0.05 µg/kg/min based on the previous patient’s response. The ED50 of phenylephrine infusion for preventing spinal-induced hypotension for cesarean delivery was estimated using a modified up-down sequential analysis, with probit analysis applied as a backup sensitivity analysis.
Results
The ED50 values for preventing spinal anesthesia-induced hypotension were 0.30 µg/kg/min (95% CI, 0.29–0.32 µg/kg/min) for group 10, and 0.19 µg/kg/min (95% CI, 0.16–0.22 µg/kg/min) for group 20, respectively. The estimated relative potency for phenylephrine in group 10 compared to group 20 was 1.52 (95%CI, 1.24–1.97), showing a significant difference in the ED50 values between the two groups.
Conclusion
This study found that a higher crystalloid co-loading rate significantly reduces prophylactic phenylephrine requirement for preventing spinal anesthesia induced hypotension.
Trials registration
https://www.chictr.org.cn/showproj.html?proj=125918 (Trial number: ChiCTR2100048002).
Keywords: Cesarean delivery, Spinal anesthesia, Hypotension, Crystalloid, Phenylephrine
Background
Spinal anesthesia and combined spinal-epidural anesthesia are the most common methods for cesarean delivery, but they are frequently associated with hypotension. Prophylactic vasopressor infusion and fluid management are considered effective strategies to prevent this complication [1–5]. However, there is limited research on the interplay between volume therapy and prophylactic vasopressor infusion in preventing spinal anesthesia-induced hypotension during cesarean delivery [5].
The optimum fluid volume for preventing spinal anesthesia-induced hypotension remains unclear, especially in conjunction with prophylactic phenylephrine. It is widely accepted that spinal anesthesia leads to hypotension primarily through sympathetic block-induced vasodilation, rather than an absolute decline in blood volume. Thus, in the context of anesthesia, prioritizing the correction of hypotension necessitates the use of vasopressors to enhance vascular resistance rather than relying solely on fluids for blood volume replenishment. We hypothesized that increasing the volume of crystalloid solution would not significantly reduce the need for prophylactic phenylephrine. The key question is whether administering an adequate volume of fluids during cesarean delivery is necessary when using preventive vasopressor therapy. In this study, we aimed to compare the median effective dose (ED50) of phenylephrine for preventing spinal anesthesia-induced hypotension when co-loading with 10 or 20 mL/kg/h of crystalloid solution, with the null hypothesis that the values of ED50 may be similar between the two groups.
Methods
Ethics
The Ethical Committee of Jiaxing University Affiliated Women and Child Hospital approved this study and issued the certificate (No. 2021-19) on May 21, 2021. Before the initiation of patients’ enrollment, the clinical trial was registered on the Chinese Clinical Trial Register on June 28, 2021 (ChiCTR2100048002, https://www.chictr.org.cn/showproj.html?proj=125918). All patients involved in this study provided written informed consent and all of them signed a written informed consent. Patient enrollment began on July 1, 2021.
Design
This was a prospective, double-blinded, randomized, dose-finding study.
Subjects and setting
Eighty patients, classified as American Society of Anesthesiologists physical status II, with a single, full-term pregnancy (gestation age ≥ 37 weeks), scheduled for elective cesarean delivery under combined spinal-epidural anesthesia, were recruited. Patients were excluded if they had contraindications to regional anesthesia, a body mass index (BMI) greater than 35 kg/m2, hypertension or preeclampsia, diabetes or gestational diabetes mellitus (GDM), or known fetal distress (fetal heart rate < 110 beats/min).
Study protocol
No premedication was administered. Upon arrival in the operating room, intravenous access was established in the patient’s left upper limb vein, and standard monitoring, including non-invasive blood pressure measurement, electrocardiogram recording, and pulse oximetry, was applied. After a brief rest period, baseline values for systolic blood pressure (SBP) and heart rate (HR) were calculated by averaging three consecutive measurements taken at three-minute intervals. Patients were then randomly assigned to receive either 10 mL/kg/h or 20 mL/kg/h of warmed Lactated Ringer’s solution at a temperature of 37°C, using a computer-generated randomized sequence using Microsoft Excel (Microsoft Corporation, Redmond, WA, United States). The infusion rates were determined based on our established clinical protocol, whereby a standard volume of 750–1500 mL of Lactated Ringer’s solution was routinely administered during cesarean delivery. Then the randomized sequence was concealed in numbered opaque envelopes, opened sequentially, enrolling one patient at a time. An anesthesia assistant aware of the patients’ grouping, adjusted the infusion rate at either 10 mL/kg/h or 20 mL/kg/h based on patient allocation but did not further participate in patient care or data collection. Anesthesia was administered, and data collected by three anesthetists (XM Zhang, J Qian, and L Liu) who were blinded to patient grouping.
Combined spinal-epidural anesthesia was completed using a needle-through-needle technique at the estimated L3–4 vertebral interspace under local anesthesia with 5 mL 2% lidocaine, while the patient was positioned on their left side. After confirming clear cerebrospinal fluid flow, 10 mg of hyperbaric bupivacaine and 5 µg of sufentanil were injected intrathecally over 15 s. An epidural catheter was then inserted approximately 3–4 cm into the epidural space, and the patient’s position was adjusted to supine with a slight tilt (approximately 15 degrees) using a wedge under the right buttock. The catheter was gently aspirated and observed for the presence of blood or CSF and was then flushed with 3 mL saline. The prepared solution was administered via infusion pump at a pre-determined rate based on patient grouping. To maintain blinding, the infusion pump surface was covered by an opaque paper.
Each patient received a preventive infusion of phenylephrine (5 mg diluted in 50 mL saline) via a syringe pump, starting simultaneously with the intrathecal injection. According to our previous reports, in the first patient in the two groups, the initial infusion rate was initiated at 0.5 µg/kg/min and sustained until infant delivery [6]. To treat hypotension (defined as a reduction in SBP below 80% of its baseline level), a 50 µg intravenous bolus dose of phenylephrine was administered. For hypertension (characterized by an increase in SBP exceeding 120% of its baseline measurement), we administer the phenylephrine infusion and resume it once the SBP drops below 120% of the baseline value. Bradycardia, characterized by a heart rate below 50 beats/min, was treated with 0.5 mg intravenous atropine if accompanied by hypertension or stopping phenylephrine infusion if hypotension was absent.
An effective infusion rate of prophylactic phenylephrine was defined as no episodes of hypotension occurring from the time of intrathecal injection until infant delivery [3, 6]. If hypotension occurred during this period, the infusion dose was deemed ineffective. The dose of phenylephrine infusion for each subsequent patient was adjusted on the patient’s previous response to phenylephrine (effective or ineffective), using the traditional up-and-down allocation method [6, 7]. If the previous patient responded effectively to the dose rate, it was reduced by 0.05 µg/kg/min for the next patient. If the dose was ineffective for the previous patient, it was increased by 0.05 µg/kg/min for the next patient. The study duration was from intrathecal injection to infant delivery. The postpartum infusion rate was adjusted based on the variation of SBP by an independent anesthesiologist uninvolved in the study.
The primary outcome measurement of this study was the effective or ineffective infusion rate of prophylactic phenylephrine. Secondary outcomes included the incidence of side effects (hypotension, hypertension, bradycardia, shivering, and nausea and vomiting), total infusion of phenylephrine and lactated Ringer’s solution administered during the study period, neonatal outcomes (Apgar scores at 1 and 5 min after delivery, umbilical arterial blood pH and lactic acid), surgical times, and anesthesia characteristics.
Statistical analysis
The recommended sample size for each group was determined to be 40 subjects, based on previous studies that indicated a range of 20–40 subjects are typically required to obtain a reliable estimate of the ED50 using the up-and-down method in most realistic scenarios [8, 9]. Additionally, data from our previous study on the ED50 of phenylephrine [6] for spinal anesthesia during cesarean delivery suggested that a minimum of 12 subjects per group would provide at least 90% statistical power to detect a difference of 15% difference in the ED50 values for phenylephrine between the groups, with an alpha level of 0.05.
The Kolmogorov-Smirnov test was used to assess the normality of the distribution for continuous variables. Normally distributed data were reported as mean ± standard deviation (SD) and analyzed using the student t-test to compare two groups. Data that did not follow a normal distribution were reported as median and interquartile range (IQR) and analyzed using the Mann-Whitney U test for comparing two groups. Categorical variables were expressed as numerical values and percentage form and analyzed using the χ2 test.
The ED50 values for phenylephrine infusion rate were calculated by averaging the midpoint values from pairs of successive parturients where an ineffective response was followed by an effective response (crossover), using the modified up-and-down method, as previously explained in the literature [6, 7, 10]. To estimate the 95% confidence interval (CI) and standard error for ED50 values, we used the methodology proposed by Choi [11]. Probit regression analysis was also performed as a sensitivity analysis, where the numbers of “effective” and “ineffective” responses were tallied for each dose category within each group. The relative potency of the ED50 values between the two groups, along with their corresponding 95% confidence intervals, was determined using Fieller’s method, which was used to calculate the ratio and difference between the two ED50 values [8]. All data analyses were conducted using GraphPad Prism 5.0 (GraphPad Software Inc, San Diego, CA) and IBM SPSS Statistics for Windows 22.0 (IBM Corp, Armonk, NY). Statistical significance was determined at a threshold of P < .05.
Results
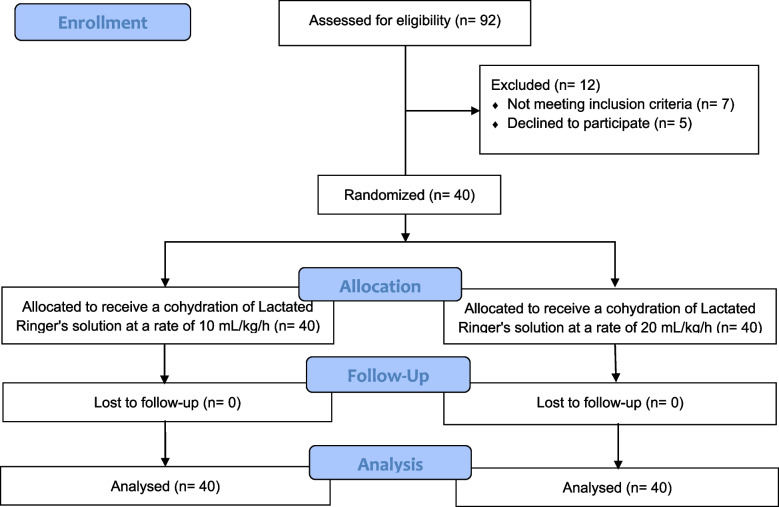
A total of 92 patients were assessed for eligibility. Five declined to participate, and seven did not meet the inclusion criteria. Finally, 80 parturients provided written informed consent and were allocated into two groups: one receiving 10 mL/kg/hour and the other 20 mL/kg/hour of Lactated Ringer’s solution. The 80 parturients were included in the final statistical analysis. The Consolidated Standards of Reporting Trials (Consort) flow is presented in Fig. 1. Demographic and baseline characteristics of the patients are shown in Table 1.
Fig. 1.
CONSORT flow diagram
Table 1.
Demography data, sensory block level, dose of phenylephrine and Lactated Ringer’s solution, and surgical times
| Characteristic | Group 10 | Group 20 | P value |
|---|---|---|---|
| Age (yrs) | 31.3 ± 4.3 | 30.2 ± 3.6 | 0.23 |
| Height (cm) | 159.4 ± 4.5 | 160.1 ± 4.2 | 0.46 |
| Weight (kg) | 67.7 ± 7.4 | 66.6 ± 5.8 | 0.46 |
| Gestational age (weeks) | 38.7 ± 0.7 | 38.6 ± 0.8 | 0.65 |
| Sensory block level (dermatome) | T5 (T5- T5) | T5 (T5- T5) | 0.34 |
| Total dose of phenylephrine used (µg) | 376 µg (299–523 µg) | 276 µg (170–414 µg) | < 0.001 |
| Total volume of Lactated Ringer’s solution administered (mL) | 178 mL (146–210 mL) | 330 mL (304–442 mL) | < 0.001 |
| Time from spinal induction to delivery (min) | 16.0 (14.0–18.0) | 15.0 (13.0–19.0) | 0.96 |
| Surgery time (min) | 49.5 (40.8–60.0) | 49.0 (43.3–60.0) | 0.79 |
Data are presented as mean (SD) or median (interquartile range)
The phenylephrine dose sequences administered to patients are presented in Fig. 2. The ED50 values were 0.30 µg/kg/min (95% CI, 0.29–0.32 µg/kg/min) for group 10, and 0.19 µg/kg/min (95% CI, 0.16–0.22 µg/kg/min) for group 20. The estimated relative potency for phenylephrine in group 10 compared to group 20 was determined to be 1.56 (95%CI, 1.31–1.93), indicating a significant difference in ED50 values between the two groups. Probit regression analysis revealed ED50 values of 0.29 µg/kg/min (95% CI, 0.24–0.34 µg/kg/min) for group 10, and 0.18 µg/kg/min (95% CI, 0.12–0.23 µg/kg/min) for group 20. The dose-response curves for prophylactic phenylephrine infusion rate to prevent spinal-induced hypotension, as simulated by probit regression, are presented in Fig. 3. The total consumption dose of phenylephrine was higher in group 10 with a (median of 376 µg (IQR) (299–523 µg), compared with 276 µg (170–414 µg) in group 20 (P < .001). The total infusion of Lactated Ringer’s solution was higher in group 20 with a median) of 330 mL (IQR) (304–442 mL), compared with 178 mL (146–210 mL) in group 10 (P < .001).
Fig. 2.
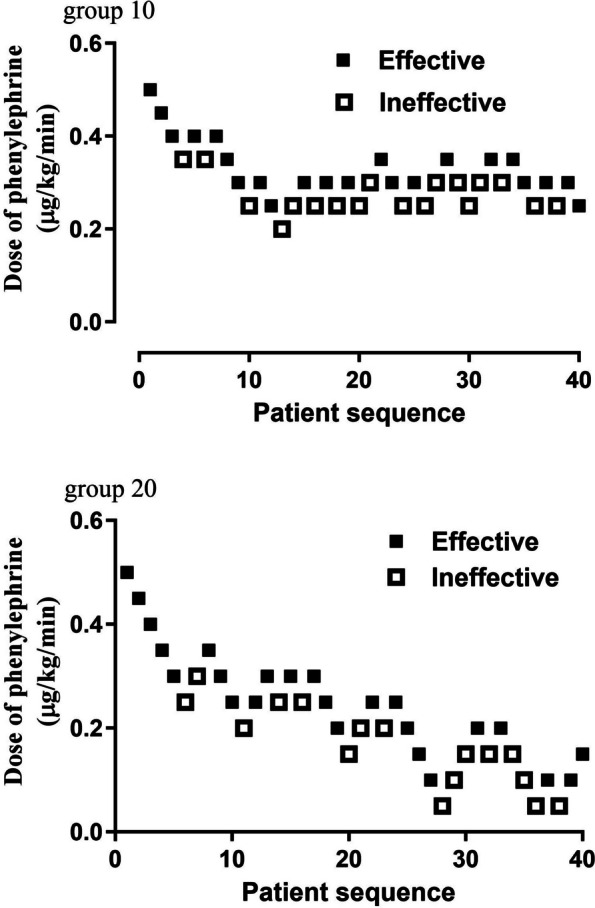
Individual response to phenylephrine infusion at corresponding doses (μg/kg/min). The ED50 of phenylephrine infusion rate, calculated using up-down analysis was 0.30 μg/kg/min (95% CI, 0.29- 0.32 μg/kg/min) for group 10, and 0.19 μg/kg/min (95% CI, 0.16- 0.22μg/kg/min) for group 20. The solid lines represent the ED50 values, while the dashed lines represent the 95% CI. Abbreviations: CI, confidence interval; ED50, effective dose in 50% of subjects
Fig. 3.
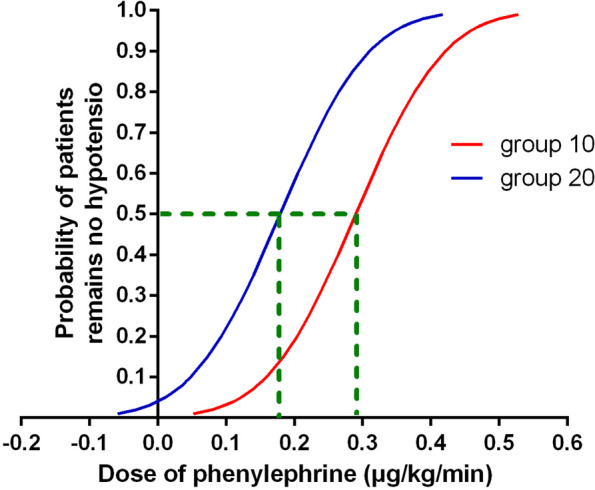
Dose-response curve of prophylactic phenylephrine infusions to prevent hypotension was plotted by estimating the probabilities of effective response (ranging from 1% to 99%) against the corresponding phenylephrine infusion rate, which was calculated using probit analysis
Side effects and neonatal outcomes are summarized in Table 2. Only one patient in group 20 experienced bradycardia, and there was no significant difference between the two groups in the incidence of maternal bradycardia (P = .31). No significant differences were found between the two groups regarding other side effects. Neonatal outcomes, including Apgar score, pH, and acid values of umbilical artery blood, were similar between the two groups.
Table 2.
Incidence of side effects and neonatal outcomes
| Characteristic | Group 10 | Group 20 | P value |
|---|---|---|---|
| Hypotension | 18 (45%) | 16 (40%) | 0.65 |
| Hypertension | 1 (2.5%) | 0 (0%) | 0.31 |
| Bradycardia | 0 (0%) | 1 (2.5%) | 0.31 |
| Shivering | 4 (10%) | 2 (5%) | 0.40 |
| Nausea | 4 (10%) | 2 (5%) | 0.40 |
| Vomiting | 1 (2.5%) | 0 (0%) | 0.31 |
| 1-min Apgar score | 9 (9–10) | 9 (9–9) | 0.11 |
| 5-min Apgar score | 10 (10–10) | 10 (10–10) | 0.57 |
| Lactic acid | 1.8 (1.5–2.1) | 1.7 (1.5-2.0) | 0.26 |
| Umbilical arterial blood pH | 7.29 ± 0.05 | 7.28 ± 0.05 | 0.48 |
Data are presented as number (%), mean (SD) or median (interquartile range)
Discussion
In this study, we observed a reduction of approximately 37% in the ED50 of phenylephrine infusion rate required to prevent spinal anesthesia-induced hypotension when co-hydration with a crystalloid solution was increased from 10 mL/kg/h to 20 mL/kg/h in women undergoing elective cesarean delivery. This suggests that the dose of prophylactic phenylephrine is affected by the volume of crystalloid coloading. No significant differences were observed in the incidence of side effects and neonatal outcomes between the two volume groups.
Existing literature indicates that crystalloid coloading, colloid preloading or coloading, and prophylactic vasopressor infusion are effective therapeutic strategies for preventing spinal anesthesia-induced hypotension during cesarean delivery [1, 5, 12, 13]. However, limited research has been conducted on the impact of varying fluid therapy volumes on the dose-response relationship of vasopressors. In this study, we were the first to compare the effects of two different crystalloid volumes on the required dose of phenylephrine infusion for preventing spinal anesthesia-induced hypotension. Our results demonstrate that a higher increase in volume of fluid administration is associated with a reduced requirement for vasopressors to prevent post-spinal hypotension. This suggests a negative correlation between the volume of fluid administered and vasopressor dosage infusion when both strategies are applied to prevent spinal anesthesia-induced hypotension under the conditions of our study.
Administering phenylephrine infusion to prevent or bolus treatment of spinal anesthesia-induced hypotension is dose-dependent and can lead to bradycardia, which may reduce cardiac output. Therefore, reducing the dosage of prophylactic phenylephrine offers valuable clinical implications, although this is not evidenced by the results of our study, which may be due to the sample size. Nevertheless, we found a significant 37% reduction in phenylephrine dosage when the crystalloid volume was increased from 10 to 20 mL/kg/h. In this study, patients in the 20 mL/kg/h group were coloaded with 304 to 442 mL of crystalloid over 13 to 19 min before neonatal delivery, which is less than previously reported volumes (1000–2000 mL) [4, 14–16]. Our findings would provide evidence that a negative correlation between cohydration volume and prophylactic vasopressor dosage exists, caution should be exercised as only two volume points were observed in this study, and further studies in this context are warranted.
The optimum dose of prophylactic phenylephrine infusion to prevent spinal anesthesia-induced hypotension during cesarean delivery remains controversial. Allen et al. [4] compared four fixed phenylephrine infusion doses of 25, 50, 75, or 100 µg/min with a 2-L fluid co-load before delivery, finding that 25 to 50 µg/min was superior to 75, or 100 µg/min. In our previous dose-response study, when combined with a co-loading of 15 mL/kg lactated Ringer’s solution over 20–30 min, we found the optimal initial dosage to be 0.54 µg/kg/min.3 The phenylephrine infusion dosage for the prevention of spinal anesthesia-induced hypotension in cesarean delivery is influenced by several factors, including the dosage of spinal local anesthetics, sensory block level, and prophylactic use of ondansetron [6]. Our findings indicate that prophylactic phenylephrine dose is also influenced by fluid volume, which may explain some of the discrepancies in previously suggested optimal infusion doses of prophylactic phenylephrine.
The ideal fluid volume to prevent hypotension remains unclear, especially when prophylactic vasopressors are used [5]. Previous studies have employed fixed volumes ranging from 500 mL to 2000 mL [4, 14–16]. However, the determination of volume relationship for crystalloid or colloid co-loading has not been established in cesarean delivery. This may be because most hemodynamic effects from sympathetic blockade occur within the first 5 to 7 min following intrathecal injection [5], meaning additional fluid administration beyond this point may offer little benefit. Practically, a moderate or a relatively lower volume of coloading is sufficient, especially when combined with a prophylactic vasopressor, and administering excessive intravenous fluids for an extended period may lead to negative outcomes following cesarean delivery. Given that spinal anesthesia primarily causes sympathetic block-induced vasodilation and relative hypovolemia, we propose that an appropriate volume of co-hydration, combined with prophylactic vasopressor infusion, is important for the management of post-spinal hemodynamics. However, the lack of a direct comparison between large and small fluid volumes, when combined with prophylactic vasopressors, limits the validity of our suggestion. Hence, further studies comparing these approaches are necessary to confirm our hypothesis. Additionally, optimizing the balance between the advantages and disadvantages of liquid volume and prophylactic vasopressor dosage to maximize patient outcomes would be highly valuable for clinical application, as our study suggests an interactive relationship between these two factors.
Some may argue that colloid co-hydration is superior to crystalloid in preventing spinal anesthesia-induced hypotension in cesarean delivery [17]. However, a study comparing colloid or crystalloid co-loading during cesarean delivery, with prophylactic phenylephrine infusion, found no advantage to using colloid over crystalloid [18]. Moreover, the use of colloids carries risks, including increased costs, allergic reactions, kidney injury, and increased mortality. For these reasons, we selected crystalloid coloading in our study. Nonetheless, further clinical research is needed to compare the efficacy of crystalloid and colloid co-hydration in preventing spinal anesthesia-induced hypotension during cesarean delivery.
We acknowledge several limitations in this study. First, we determined and compared only ED50 values for phenylephrine administered at varying volumes of crystalloid using the conventional up-and-down allocation method. It is important to note that in the real world, anesthesia providers tend to prefer doses such as ED90 or ED95, and even ED99, which are more clinically relevant than ED50. Hence, further studies should aim to establish these doses for clinical reference. Second, our findings cannot be applied to high-risk or emergency cesarean deliveries due to the stringent criteria used for inclusion and exclusion. Finally, the secondary outcomes, such as side effects in this study, should be interpreted with caution, as our sample size was primarily determined based on the main outcomes, which may not have been sufficient to detect statistically significant differences in the exploratory outcomes.
In summary, under the conditions of this study, we found that a higher rate of crystalloid co-loading reduced the prophylactic phenylephrine requirement for preventing spinal anesthesia-induced hypotension in cesarean delivery.
Acknowledgements
We express our sincere appreciation to all the personnel in the operating room who provided assistance during this study.
Abbreviations
- ED50
Median effective dose
- BMI
Body mass index
- SD
Standard deviation
- SBP
Systolic blood pressure
- HR
Heart rate
- IQR
Interquartile range
- CI
Confidence interval
Authors’ contributions
XMZ, JQ and LL helped in conducting the study and collecting the data; XMZ, YPS and FX helped in writing and preparing the manuscript. All authors reviewed the manuscript.
Funding
Health Program of Zhejiang Province (2022KY1264).
Data availability
All data generated or analysed during this study are included in this published article.
Declarations
Ethics approval and consent to participate
The Ethical Committee of Jiaxing University Affiliated Women and Child Hospital approved this study and provided the certificate (No. 2021-19) on May 21, 2021. All patients involved in this study were informed and all of them signed a written informed consent. Initiation of patient enrollment was on July 1, 2021.
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yue-Ping Shen, Email: 378425362@qq.com.
Fei Xiao, Email: 13706597501@163.com.
References
- 1.Massoth C, Töpel L, Wenk M. Hypotension after spinal anesthesia for cesarean section: how to approach the iatrogenic sympathectomy. Curr Opin Anaesthesiol. 2020;33:291–8. [DOI] [PubMed] [Google Scholar]
- 2.Kumari K, Chaudhary K, Sethi P, et al. Norepinephrine versus phenylephrine for postspinal hypotension in parturients undergoing cesarean section: a systematic review and meta-analysis. Minerva Anestesiol. 2022;88:1043–56. [DOI] [PubMed] [Google Scholar]
- 3.Xiao F, Shen B, Xu WP, Feng Y, Ngan Kee WD, Chen XZ. Dose-response study of 4 weight-based phenylephrine infusion regimens for preventing hypotension during cesarean delivery under combined spinal-epidural anesthesia. Anesth Analg. 2020;130:187–93. [DOI] [PubMed] [Google Scholar]
- 4.Allen TK, George RB, White WD, Muir HA, Habib AS. A double-blind, placebo-controlled trial of four fixed rate infusion regimens of phenylephrine for hemodynamic support during spinal anesthesia for cesarean delivery. Anesth Analg. 2010;111:1221–9. [DOI] [PubMed] [Google Scholar]
- 5.Rijs K, Mercier FJ, Lucas DN, Rossaint R, Klimek M, Heesen M. Fluid loading therapy to prevent spinal hypotension in women undergoing elective cesarean section: network meta-analysis, trial sequential analysis and meta-regression. Eur J Anaesthesiol. 2020;37:1126–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Xiao F, Wei C, Chang X, et al. A prospective, randomized, double-blinded study of the effect of intravenous ondansetron on the effective dose in 50% of subjects of prophylactic phenylephrine infusions for preventing spinal anesthesia-induced hypotension during cesarean delivery. Anesth Analg. 2020;131:564–9. [DOI] [PubMed] [Google Scholar]
- 7.Dixon WJ. Staircase bioassay: the up-and-down method. Neurosci Biobehav Rev. 1991;15:47–50. [DOI] [PubMed] [Google Scholar]
- 8.Pace NL, Stylianou MP. Advances in and limitations of up-and-down methodology: a précis of clinical use, study design, and dose estimation in anesthesia research. Anesthesiology. 2007;107:144–52. [DOI] [PubMed] [Google Scholar]
- 9.Stylianou M. Sequential analysis of Durham and Flournoy’s biased coin design for phase I clinical trials. Washington, DC: American University; 2000. p. 1–178. [Google Scholar]
- 10.Burlacu CL, Gaskin P, Fernandes A, Carey M, Briggs L. A comparison of the insertion characteristics of the laryngeal tubeand the laryngeal mask airway: a study of the ED50 propofol requirements. Anaesthesia. 2006;61:229–33. [DOI] [PubMed] [Google Scholar]
- 11.Choi SC. Interval estimation of the LD50 based on an up-anddown experiment. Biometrics. 1990;46:485–92. [PubMed] [Google Scholar]
- 12.Chooi C, Cox JJ, Lumb RS, et al. Techniques for preventing hypotension during spinal anaesthesia for cesarean section. Cochrane Database Syst Rev. 2020;7:CD002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Singh PM, Singh NP, Reschke M, Ngan Kee WD, Palanisamy A, Monks DT. Vasopressor drugs for the prevention and treatment of hypotension during neuraxial anaesthesia for cesarean delivery: a Bayesian network meta-analysis of fetal and maternal outcomes. Br J Anaesth. 2020;124:e95–107. [DOI] [PubMed] [Google Scholar]
- 14.McDonald S, Fernando R, Ashpole K, Columb M. Maternal cardiac output changes after crystalloid or colloid coload following spinal anesthesia for elective cesarean delivery: a randomized controlled trial. Anesth Analg. 2011;113:803–10. [DOI] [PubMed] [Google Scholar]
- 15.Bottiger BA, Bezinover DS, Mets B, et al. Phenylephrine infusion for spinal-induced hypotension in elective cesarean delivery: does preload make a difference? J Anaesthesiol Clin Pharmacol. 2016;32:319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mercier FJ, Diemunsch P, Ducloy-Bouthors AS, et al. 6% hydroxyethyl starch (130/0.4) vs Ringer’s lactate preloading before spinal anaesthesia for cesarean delivery: the randomized, double-blind, multicentre CAESAR trial. Br J Anaesth. 2014;113:459–67. [DOI] [PubMed] [Google Scholar]
- 17.Fitzgerald JP, Fedoruk KA, Jadin SM, Carvalho B, Halpern SH. Prevention of hypotension after spinal anaesthesia for cesarean section: a systematic review and network meta-analysis of randomised controlled trials. Anaesthesia. 2020;75:109–21. [DOI] [PubMed] [Google Scholar]
- 18.Park SK, Park DN, Kim YW, et al. Colloid coload versus crystalloid coload to prevent maternal hypotension in women receiving prophylactic phenylephrine infusion during cesarean delivery: a randomised controlled trial. Int J Obstet Anesth. 2022;49:103246. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analysed during this study are included in this published article.