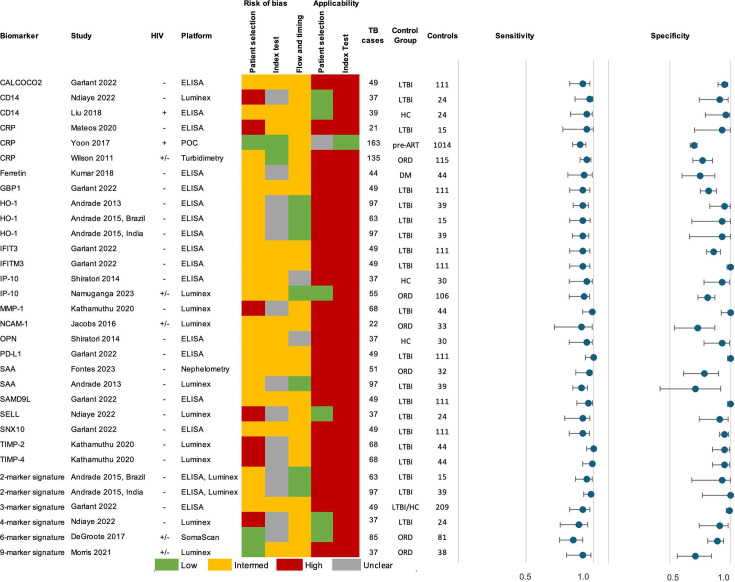
Fig 3.
Summary of adult PTB biomarkers and signatures meeting TPP criteria. Control groups: DM = diabetes mellitus, HC = healthy control, LTBI = latent TB infection, ORD = other respiratory disease, pre-ART = before initiation of anti-retroviral therapy. 2-marker signatures: HO-1, MMP-1; 3-marker signature: CALCOCO2, IFITM3, SAMD9L; 4-marker signature: CLEC3B, ECM1, IP-10, SELL; 6-marker signature: SYWC, kallistatin, C9, gelsolin, testican-2, aldolase C; 9-marker signature: fibrinogen, α-2-M, CRP, MMP-9, TTR, CFH, IFN-γ, IP-10, TNF. The reference standard results for risk of bias and applicability were low for all studies.