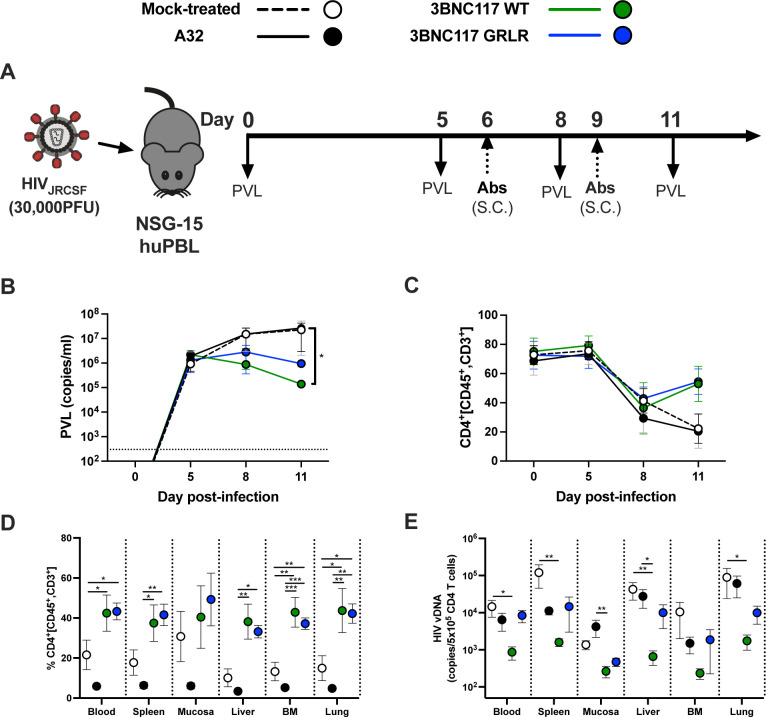
Fig 7.
A32 nnAb does not impact viral replication or the size of the reservoir in vivo. (A) Experimental outline. NSG-15-Hu-PBL mice were infected with HIV-1 JRCSF intraperitoneally. At days 6 and 9 post infection, mice were administered 1.5 mg of A32 or 3BNC117 (WT or GRLR) mAb subcutaneously (S.C.). (B) Mice were bled routinely for plasma viral load (PVL) and flow cytometry analysis. PVL levels were measured by quantitative real-time PCR (limit of detection = 300 copies/mL, dotted line). (C) Percentage of CD4+ T cells in peripheral blood was evaluated by flow cytometry. At least six mice were used for each treatment. (D, E) Tissues of JRCSF-infected NSG-15 hu-PBL mice, treated or not with A32 or 3BNC117 (WT or GRLR), were harvested at day 11. (D) Percentage of CD4+ T cells was evaluated by flow cytometry. (E) CD4+ T cells were isolated for real-time PCR analysis of HIV DNA. Each dot represents the mean values ± SEM. S.C., subcutaneous; I.P., intraperitoneal; BM, bone marrow; mock treated, no antibody. Statistical significance was tested using one-way ANOVA with a Holm–Sidak post-test or a Kruskal–Wallis test with a Dunn’s post-test (*P < 0.05,**P < 0.01,***P < 0.001).