Abstract
Background
Heat stress (HS) commonly occurring in summer has gradually become a factor threatening the reproductive performance of male dairy goats by reducing their fecundity. Despite the melatonin is applied to relieve HS, it is still unclear whether melatonin protects against reproductive damage induced by HS in dairy goats and how it works. The purpose of the present study is to evaluate the role of melatonin in alleviating HS-induced spermatogenesis dysfunction in male dairy goats and further explore its mechanism.
Results
HS impaired spermatogenesis, sperm formation in the testes, and sperm maturation in the epididymis of dairy goats, resulting in decreased sperm quality. Melatonin rescued the decrease of sperm quality induced by HS via decreasing inflammatory and oxidative stress levels in testicular tissue and enhancing intercellular barrier function within the testes. Amplicon-based microbiota analysis revealed that despite gut microbiota differences between melatonin-treated dairy goats and NC dairy goats to some extent, melatonin administration tends to return the gut microbiota of male dairy goats under HS to the levels of natural control dairy goats. To explore whether the protective role of melatonin in sperm quality is mediated by regulating gut microbiota, fecal microbiota of HS dairy goats with or without melatonin treatment were transferred to HS mice, respectively. We found HS mice that had received fecal bacteria of HS dairy goats experienced serious testicular injury and dyszoospermia, while this phenomenon was ameliorated in HS mice that had received fecal bacteria of dairy goats treated with melatonin, indicating melatonin alleviates HS-induced spermatogenic damage in a microbiota dependent manner. We further found that the testicular tissue of both HS dairy goats and mice transplanted with HS dairy goat feces produced large amounts of arachidonic acid (AA)-related metabolites, which were closely associated with semen quality. Consistently, supplementation with AA has been shown to elevate the levels of inflammation and oxidative stress in the testicular tissue of mice, disrupting intercellular connections and ultimately leading to spermatogenic disorders.
Conclusion
This study has revealed that melatonin can effectively alleviate spermatogenic disorders in dairy goats caused by HS. This beneficial effect was primarily achieved through the modulation of gut microbiota, which subsequently inhibited the excessive synthesis of AA in testicular tissue. These discoveries are of great significance for preventing or improving the decline in male livestock reproductive performance caused by HS, enhancing the reproductive efficiency of elite male breeds, and ultimately improving the production efficiency of animal husbandry.
Video Abstract
Supplementary Information
The online version contains supplementary material available at 10.1186/s40168-024-01942-6.
Keywords: Melatonin, Heat stress, Gut microbiota, Arachidonic acid, Male reproduction
Background
Sustainable spermatogenesis, as the index of reproduction performance of male dairy goats, works best at temperatures between 2 ℃ and 7 ℃ below body temperature. High temperatures naturally occurring in summer were demonstrated to damage the production and reproductive performance of dairy goats, manifested by decreased sperm motility, increased dead sperm, and abnormal sperm, which poses a grave threat to the sustainability of the livestock industry [1].
Given that HS could disrupt the function and shape of the supporting cells and break the tight connection between the cells and the blood-testicular barrier (BTB), leading to infertility and germ cell death [2–5]. For instance, our previous study displayed that HS caused testicular germ cell disorder and loss, spermatocyte apoptosis, endoplasmic reticulum stress, mitochondrial swelling, and structural destruction, increasing the secretion of endogenous pyrogen in mice [6, 7]. On the other hand, HS is accompanied by gut microbiome dysbiosis, which is proposed to threaten host physiological health such as reproductive damage by inducing the development of multiple microbiomes and/or their metabolism [6, 8]. The gut microbiota of mice dosed with alginate oligosaccharides enhances spermatogenesis by benefiting the gut microbial community of the recipients [9]. Consistently, the microbially derived metabolite 3-hydroxyphenylacetic acid promotes spermatogenesis in 18–20 months mice [10]. In conclusion, the gut testis axis can affect male sterility to some extent. A current study reported that the enrichment of Gram-negative microorganisms in the gut enhances the conversion of arachidonic acid (AA), a 20-carbon fatty acid essential to cell membrane phospholipids, to PGE2, which is a potent pro-inflammatory agent linked to semen quality [11, 12]. These studies indicated that the gut testis axis can affect male sterility to some extent. Thus, we posit that the close correlation between semen quality and AA metabolism is likely influenced by the intestinal microbiota.
Melatonin (N-acetyl-5-methoxytryptamine), a neuroendocrine hormone in the hypothalamus, is involved in the circadian rhythm regulation of physiological and neuroendocrine functions [13–19]. The beneficial effects of melatonin in immune response, inflammation, diabetes, obesity, lipid metabolism, and metabolic syndrome are gradually demonstrated by investigators [20–26]. The gut microbiota plays an important role in mediating the regulation of melatonin in various host physiological functions [13–19]. In detail, Yin et al. (2018) reported that melatonin alleviated lipid metabolism disorders in mice fed a high-fat diet by reprogramming the gut microbiome [27]. Wang et al. (2023) displayed that melatonin plays a neuroprotective role in sleep-deprived cognitive impairment by reducing the levels of Aeromonas and Lipopolysaccharide (LPS) and increasing the levels of Lachnospiraceae NK4A136 and butyrate [28]. Similarly, melatonin is demonstrated to affect weight gain, intestinal morphology, and enterotoxic Escherichia coli infection in weaned mice through intestinal flora [29]. At present, investigations on melatonin in the repair effect of testicular injury caused by HS are focused on its direct effect on spermatogenic cells. However, whether melatonin prevents HS-induced reproductive injury in dairy goats by the gut-testicular axis, that is, reshaping gut microbiome and their metabolism remains unclear.
In this study, we initially explored the effect of melatonin on the reproductive performance of HS dairy goats. To detect the protective mechanism of melatonin in HS, gut microbiota reshaped by melatonin and its related testicular metabolisms were used to verify the rescued effect of melatonin in the HS model. This study provides new insight into melatonin in the mechanism of relieving male reproductive system damage caused by HS and paves the way for the potential application of melatonin to prevent and treat testicular dysfunction caused by HS in male animals.
Materials and methods
Animals
All experimental animals and procedures were approved by the Animal Ethical and Welfare Committee Ethics, Northwest A&F University, China, and performed in strict accordance with the university’s guidelines for animal research (Approval No. 201902A299). The dairy goat experiment was completed in Shaanxi Aonick Dairy Goat Breeding Co., Ltd., Fuping County, Weinan City, Shaanxi Province, China. During the experimental period, the estimated photoperiod in Fuping County, Shaanxi Province, comprised 14 h of light exposure and 10 h of darkness. Each dairy goat is fed approximately 1 kg of Total Mixed Ration daily, comprising roughage (such as alfalfa, peanut vine, and corn husk) and 0.75 kg of concentrate, which fully meets their nutritional requirements. Male ICR mice (25–30 g, 8 weeks old) purchased from Chengdu Dossy Experimental Animals Co., Ltd. were used to conduct the experimental operations. Mice were kept at a constant temperature 12 of 22 ± 2 °C on a 12-h light/dark cycle and had unrestricted access to food and water. During the experiment, animal control officers and researchers knew nothing about the grouping of test animals.
Heat stress (HS) administration
Thirty dairy goats aged between 2.5 and 4.5 years, with a weight of approximately 50 kg, were selected after they had adapted to the experimental environment for over 1 week, and were then randomly divided into the Natural Control group (NC), heat stress group (HS) and melatonin group (MT). In the MT group, dairy goats were implanted immediately behind the ears with a vehicle or 1 mg/kg melatonin agent (Regulin, France). Subsequently, both the HS group and the MT group underwent HS conditions for a duration of 21 days [30]. The heat stress model of dairy goats was established by stabilizing the Temperature-Humidity Index (THI) remained above 73 [30]. To clarify further, the heat stress model for dairy goats was established through the use of natural high temperatures. During the course of the experimental period, both the HS group and the MT group of goats were placed on an outdoor open-air sports field from 12:00 noon to 16:00 each day, allowing them to freely access water and food while being exposed to direct sunlight [1]. The ambient temperature and air humidity were measured every day during the test, and the temperature and humidity index (THI) was calculated. The THI was calculated using the following equation: THI = [0.8 × ambient temperature (°C)] + [(% relative humidity/100) × (ambient temperature − 14.4)] + 46.4 [31, 32]. Dairy goats in the NC group were tested in the same environment in autumn (non-HS state), and the test scheme remained consistent.
Fecal microbiota transfer (FMT) operation
Antibiotic cocktails (Abx) (100 mg/L penicillin, 100 mg/L neomycin, 100 mg/L metronidazole, 50 mg/L vancomycin, and 50 mg/L streptomycin) purchased from Beijing Coolaber were supplemented to mice by drinking water. Briefly, the Abx was changed daily, and the mixture was used as usual [33]. After 7 days of continuous high-dose Abx treatment, 20 mice were randomly divided into FMT (HS goat) -HS group (n = 10) and FMT (MT goat) -HS group (n = 10).
Rectal feces of dairy goats in the HS group and MT group were homogenized with LB medium, and then the fecal suspension was filtered. The mice were supplemented with fecal suspension every 2 days from 2 weeks before HS administration until the end of the experiment. Finally, mice receiving dairy goat fecal microbiota were subjected to HS at 37 ℃ in an HS chamber at constant temperature and humidity for 1 week, and samples were collected.
Arachidonic acid (AA) test in mice
To verify the damaging effect of excessive AA on male reproduction, 12 mice were divided into two groups, which were labeled as the NC group and the AA group. Then, mice were given AA (300 mg/kg) and normal saline for 28 days, respectively. After the test, sperm from the tail of the epididymis of mice and testis and epididymis samples were collected. AA oil was purchased from CABIO Biotech (Wuhan) Co., Ltd.
Semen collection and sperm quality detection
The semen of dairy goats was collected every 2–3 days during the experimental period using the false vaginal sperm collection method. The original semen was diluted 3–4 times with semen diluent and observed under a × 400 microscope. The Mylan Animal Sperm Analyzer (Songjing, China) was used to analyze sperm density, sperm motility, and sperm viability.
Mouse epididymis tail sperm Giemsa staining: fresh epididymal tissue was cut into pieces in 37 ℃ semen dilution so that sperm was completely released. Make A sperm smear, air dry, and successively add Giemsa A and phosphate buffer B (PH1793, Phygene). The sections were observed under a research-grade upright microscope (Nikon, Ni-U, Japan).
Sample collection
Testis and epididymis collected from dairy goats were cut into appropriate size tissue blocks, and then stored in 4% multi-filtered formaldehyde tissue fixative (BL539A, Biosharp) and liquid nitrogen, respectively. Feces were partly stored in liquid nitrogen for microbial sequencing and follow-up tests, and the other part was stored in a 15% glycerol LB medium for FMT administration.
Hematoxylin and eosin (H&E) staining
Testes and epididymis fixed with 4% multi-filtered formaldehyde tissue fixative were dehydrated by graded ethanol, embedded in paraffin, and sliced into 2-μm sections. The tissues were fixed in Bouin’s solution for hematoxylin and eosin (H&E) staining.
Immunofluorescence staining.
Tissues (paraffin sections, 2 µm) were dewaxed with xylene and rehydrated with gradient alcohol. Subsequently, the tissue was subjected to the EDTA antigen repair solution for 20 min, 10% fetal bovine serum incubation at room temperature for 1 h, and a specific primary antibody of ZO-1 (Abcam, ab221547) and occludin (Abcam, ab216327) staining overnight at 4° C. Then, the samples were incubated with FITC-labelled goat anti-rabbit IgG (BOSTER, ab1146) sourced from Boster at a dilution ratio of 1:200, at 4° C for 4 h. Finally, the tablets were sealed with an anti-fluorescence quencher containing DAPI (meilunbio, MA0222-2). A laser confocal microscope (Nikon, A1 + /A1R + , Japan) was observed and photographed.
Enzyme-linked immunosorbent assay (ELISA)
Testicular tissue was fully ground in the automatic sample rapid grinding instrument (Shanghai Jingxin, JXFSTPRP-48L). TNF-α, IL-6, IL-1β, AA, prostaglandin D2 (PGD2), leukotriene B4 (LTB4), and 12-hydroxy-5Z, 8Z, 10E, 14Z, eicosatetraenoic acid (12-HETE) in the supernatant were determined by goat and mouse ELISA kit (Shanghai Kexing Trading Co., Ltd.).
MDA and SOD assays
MDA and SOD assays were performed as described previously [34]. Briefly, after weighing the tissue sample, centrifuge it with 9 times the volume of normal saline homogenate at 4000 rpm for 10 min, and the supernatant is used for MDA and SOD detection. MDA levels were determined by the TBA method and SOD activity was determined by WST-1 method. The detection procedures were carried out according to the scheme provided by the Nanjing Institute of Jiancheng Bioengineering.
Western blotting
Testicular tissue was homogenized with RIPA lysis buffer (Beyotime, China) containing phosphatase inhibitors and phenylmethylsulfonyl fluoride (PMSF). Centrifuge at 12,000 rpm. The total protein in the supernatant was mixed with the sample buffer (Beyotime, China), and heated at 100 ℃ for 8 min. The proteins were separated by SDS-PAGE electrophoresis and transferred to the PVDF membrane (Millipore, USA). The proteins in the membrane were subjected to the blockade with 8% skim milk for 2 h and incubated with the primary antibody at 4 ℃ overnight and the secondary antibody, at 4 ℃ for 4 h. Immunoreactive bands were observed with ECL luminescent solution (Mishu, MI00607B) and multifunctional imaging system (SHENHUA, Hangzhou, China), and β-Actin was used as the internal reference. The information on antibodies is shown in Table S3, Supplementary information.
16S rRNA amplicon and sequencing
Collect the rectal contents, quickly freeze them in liquid nitrogen, and transfer them to a − 80° C freezer for preservation. The V3–V4 hypervariable region of the 16S rRNA gene is amplified using TransGen Biotech’s Pfu high-fidelity DNA polymerase. The sequencing procedures were completed by Shanghai Bioprofile Technology Company Ltd. In QIIME, DADA2 is invoked for quality control, denoising, merging, and chimera removal. Using the classifier -sklearn algorithm [35] based on ASV distribution, the α diversity level of each sample is evaluated. At the ASV level, the distance matrix for each sample is calculated, and unsupervised ordination, clustering, and statistical testing methods are employed to quantify the β diversity differences and their significance among different samples. Using linear discriminant analysis (LDA) effect size (LEfSe) [36] analysis, the differences in species abundance composition between samples are further evaluated to identify potential biomarker species. Based on the 16S rRNA sequencing results, microbial metabolic functions are further predicted to determine the differentially abundant biological functional pathways and the species composition of specific pathways.
LC–MS/MS analysis
Testicular tissue samples of dairy goats were prepared and deproteinized with methanol. The metabolomics analysis of testicular tissue was performed using the liquid-mass combination (LC–MS) technique from Novogene Co., Ltd. UHPLC-MS/MS analyses were performed using a Vanquish UHPLC system (ThermoFisher, Germany) coupled with an Orbitrap Q ExactiveTM HF mass spectrometer (Thermo Fisher, Germany) in Novogene Co., Ltd. (Beijing, China). These metabolites were annotated using the KEGG database (https://www.genome.jp/kegg/pathway.html), Principal components analysis (PCA) and Partial least squares discriminant analysis (PLS-DA) were performed at metaX [37]. We applied univariate analysis (t-test) to calculate the statistical significance (P-value). The metabolites with VIP > 1 and P-value < 0.05 and fold change ≥ 2 or FC ≤ 0.5 were considered to be differential metabolites. P-value < 0.05 was considered statistically significant and correlation plots were plotted by corrplot package in R language.
RNA isolation and real-time quantitative polymerase chain reaction (RT-qPCR)
Total RNA was extracted using TRIZOL reagent (TAKARA) and converted to cDNA using FastKing RT Kit (Tiangen Biotech, China) by the manufacturer’s protocols. cDNA was subjected to qPCR and gene expression was measured using SYBR Green (Vazyme, China) in accordance with the manufacturer’s protocols. The qPCR instrument was purchased from BIO-RAD (CFX Connect Optics Module, Serial NO. 788BR08727, Singapore). The primer information is shown in Table S2, Supporting Information.
Statistical analysis
Student’s t-test was used to analyze whether there were statistically significant differences between groups (P-value < 0.05). One-way analysis of variance (ANOVA) followed by Dunnett’s multiple comparison tests was used to determine whether there were significant differences between more than two treatments. Values are expressed as the mean ± standard deviation (SD) with at least three independent experiments repeated [38]. Statistical analyses were performed using SPSS 26.0, and data visualization was achieved with GraphPad Prism 8.0.2 software. A P-value < 0.05 was considered a statistically significant difference, and a P-value < 0.01 was considered a highly significant reference difference.
Results
Melatonin alleviates HS-induced decline of sperm quality in dairy goats
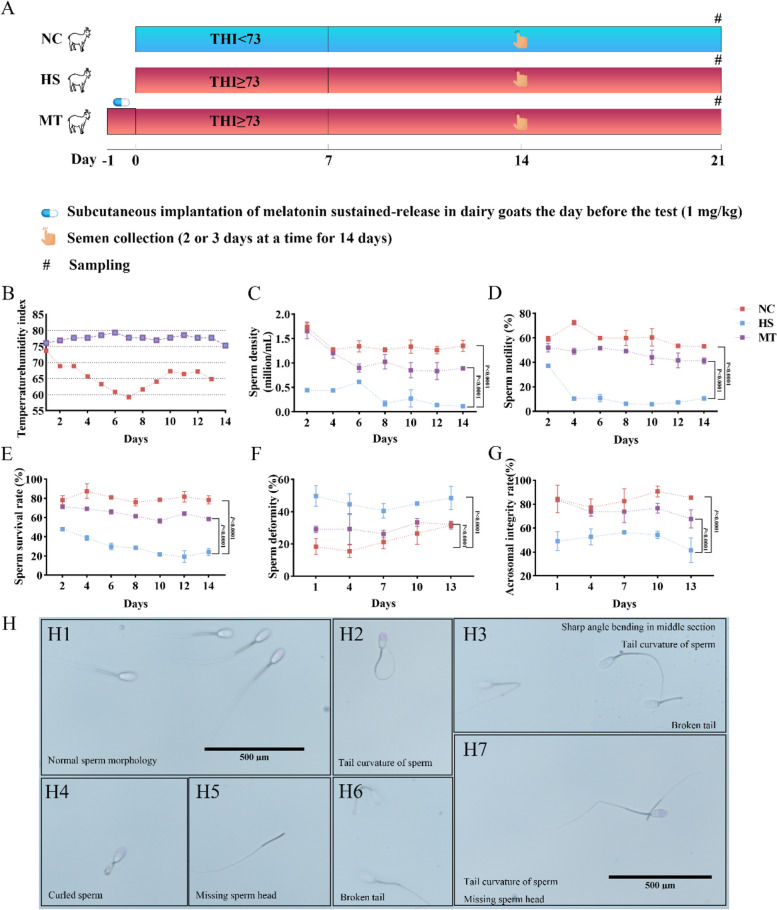
To clarify the effect of melatonin on HS-induced male reproduction of dairy goats, HS was initially verified by THI index ranging from 73 to 80, and dairy goats in the MT group were subcutaneously implanted with melatonin the day before HS (Supplementary Table S1, Fig. 1A, B). The sperm quality of dairy goats, which was evaluated by the index of sperm density (P < 0.01), motility (P < 0.01), and sperm survival rate (P < 0.01), decreased significantly in the HS group compared to the goats in the NC group. However, under the condition of pre-implantation of melatonin, the adverse effects of HS on the sperm quality of dairy goats were partially alleviated throughout the entire experimental period (Fig. 1C, D, E). Sperm quality analysis revealed a significantly higher sperm malformation (P < 0.01) (Fig. 1F) and a significantly lower acrosomal integrity (P < 0.01) (Fig. 1G) in the goats of the HS group than the goats of the NC group. In the MT group, sperm quality is restored partially to the level of the goats in the NC group. The morphological analysis of sperm by microscopy showed that the sperm of dairy goats in the HS group have a variety of morphological deformities, including missing sperm head, sharp angle bending in the neck and middle section, broken tail, and curled sperm (Fig. H1– 7). These results suggest that HS significantly increased the risk of malformed sperm and damaged the acrosomal structure of sperm, leading to a significant decline in sperm survival rate, and ultimately damaged sperm quality. Pre-treatment with a melatonin implant does protect sperm to a certain extent, stabilize sperm structure, and help sperm resist the damage caused by HS.
Fig.1.
Melatonin ameliorates the decrease in sperm quality caused by HS in dairy goats. A Schematic diagram of experimental design. NC, dairy goats in natural control group; HS, dairy goats in heat stress group; MT, HS dairy goat group pretreatment with melatonin; Dairy goats in HS/MT group (n = 10) were implanted with/without melatonin (1 mg/kg) on the day before the experiment initiation. After 7 days of HS, semen was collected once every 2–3 days to detect sperm quality, and samples were collected on the 21st day. Dairy goats in the NC group (n = 10) received the same treatment in the autumn (without HS) as the other two groups. B THI index. C Sperm density of dairy goats in each group. D Sperm motility. E Sperm survival rate. F Sperm malformation rate. G Acrosomal sperm integrity rate. H Sperm morphology of dairy goats. Scale bars = 500 µm. The statistical significance of the data in A–G was determined using two-way ANOVA
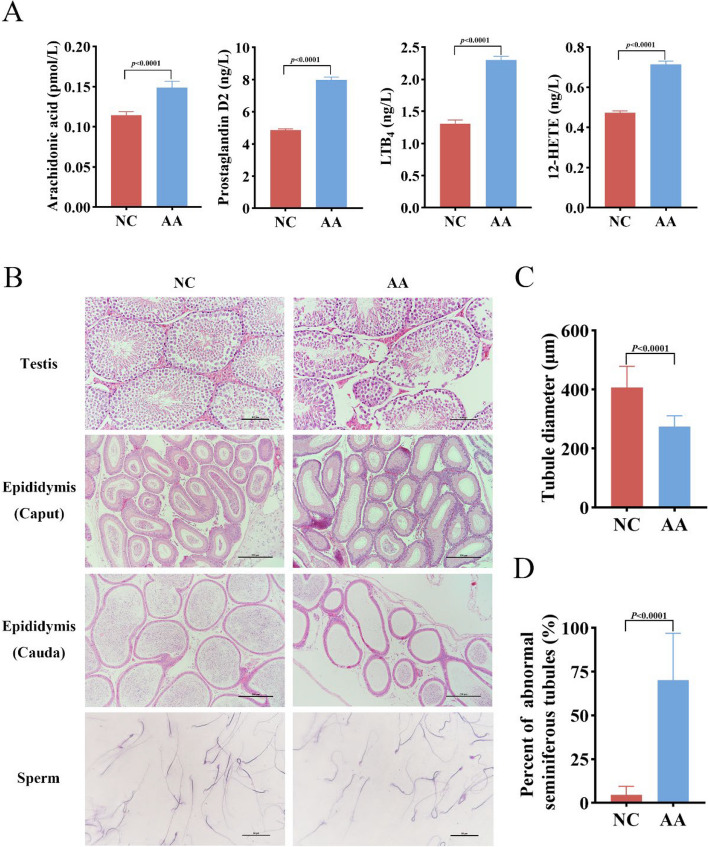
Fig. 7.
AA-induced reproductive damage of male mice. A Concentration of AA, prostaglandin D2, leukotriene B4, and 12-HETE in testis tissue of mice in NC and AA groups. B HE staining of tissue sections from testis (scale = 100 µm), head of epididymis, and tail of epididymis (scale = 250 µm) in NC and AA groups, and Giemsa staining of sperm in epididymis tail (scale = 500 µm). C Statistical diagram of spermatogenic tubule diameter. Three sections were taken from each mouse and 207 spermatogenic tubules were used to measure the diameter. D Percentage of abnormal curved seminiferous tubules. Testicular slices of mice in each group were observed under the microscope in 9 representative fields. In A, C, D, statistical significance was assessed by independent samples t-test
Melatonin protects the sperm quality of dairy goats by repairing testicular damage induced by HS
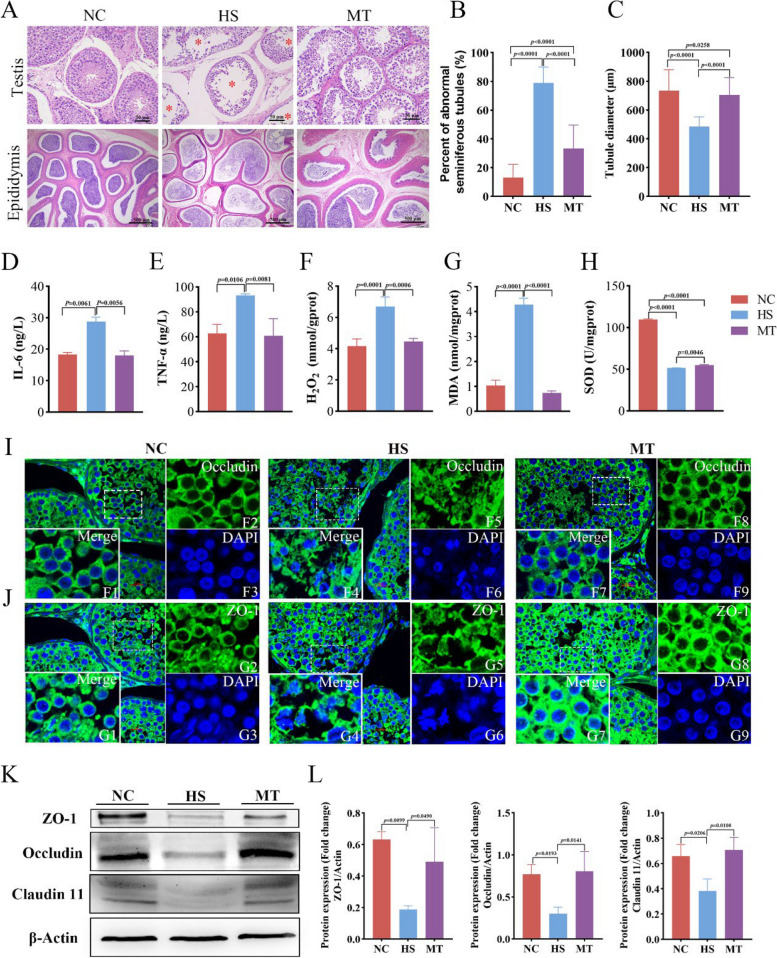
To further reveal the protective function of melatonin on sperm quality of dairy goats under HS, phenotypic analyses of the testis and epididymis of HS and MT dairy goats were performed. The testis volume of dairy goats in the HS group exhibited a slight reduction, and the spermatic cords of the testis in the HS group became thicker and rudder than those in the other two groups (Supplementary Figure S1). This is due to vasodilation and increased blood flow caused by HS, which leads to blood retention in the testicular veins of dairy goats. Histological evaluation of the transverse section of the testis showed that the testis of the HS group had structural damages, including spermatogenic tubule destruction, spermatogenic cell loss, and loose interstitial cell arrangement (Fig. 2A). The diameter of convoluted tubules decreased significantly (P < 0.01), while the number of abnormal curved seminiferous tubules increased notably (P < 0.01) (Fig. 2B, C). The quantity of mature sperm in the tail tubes of the epididymis decreased significantly. After the treatment of melatonin, the damage of testicular tissue from MT dairy goats was ameliorated obviously. Especially, in MT dairy goats, the spermatogonia were closely arranged in layers, and the number of spermatogenic cells and mature sperm was significantly increased in the tail of the epididymis (Fig. 2A). We next evaluated the effect of melatonin on HS-induced proinflammatory responses in testicular tissue of dairy goats. The results showed that compared with the NC group, the levels of TNF-α (P < 0.05) and IL-6 (P < 0.01) in the testicular tissue of dairy goats in the HS group significantly increased. In contrast, the MT group significantly reversed the increase (Fig. 2D, E). Similarly, dairy goats supplemented with melatonin reduced the increase of H2O2 (P < 0.01) and MDA (P < 0.01) in testicular tissue and the decrease of SOD (P < 0.01), suggesting the ability of melatonin to repair HS-induced oxidative stress levels in testicular tissue of dairy goats (Fig. 2F–H). The analysis of immunofluorescence results indicated that the occludin and ZO-1 staining in testicular tissue of dairy goats in the HS group showed blurred cell membrane contour, serious rupture and disorder, and decreased uniformity of karyoplasm distribution, by contrast conversely, the position and expression of occludin and ZO-1, cell morphology and gloss, and plasma membrane boundary in testicular tissue in MT group were restored to NC group level (F ig. 2I–J). The barrier integrity detection in protein levels showed that compared with the NC group, the expression of specific tight junction proteins occludin (P < 0.05), ZO-1 (P < 0.01), and Claudin 11 (P < 0.05) in testicular tissue of dairy goats in the HS group was decreased, while the expression of specific tight junction proteins in MT group slightly increased (Fig. 2K, L). These results suggested that melatonin ameliorated inflammation and oxidative stress within the testicular tissue caused by HS, and it fortified the intercellular barrier function among testicular cells. Consequently, these effects mitigated the adverse effects of HS on sperm quality, offering protection against HS-induced deterioration.
Fig. 2.
Melatonin alleviates HS-induced testicular inflammation and oxidative stress and repairs testicular barrier function in dairy goats. A Testicular morphology (scale bars = 50 µm) and epididymis morphology (scale bars = 500 µm) in different groups, and a red asterisk represents abnormal curved seminiferous tubules. B Percentage of abnormal seminiferous tubules and testicular sections of dairy goats in each group were observed under the microscope in 20 representative fields. C Statistical diagram of spermatogenic tubule diameter. Three sections were taken from each dairy goat and 163 spermatogenic tubules were used to measure the diameter. D The level of IL-6 in the testicular tissue of dairy goats in each group was detected by ELISA (n = 3). E The level of TNF-α (n = 3). F The level of H2O2 (n = 3). G The level of MDA (n = 3). H The level of SOD (n = 3). I, J Immunofluorescence images of occludin, ZO-1in testicular tissue of dairy goats in different groups. Scale bars = 20 μm. (Each dashed white box represents the enlarged position in the Merge graph in the lower left corner.) K Western blotting showing TJPs (claudins 11, occludin, and ZO-1) expression in different groups. L Densitometric data of corresponding K were normalized to β-Actin (n = 3). In B–H, L P-values were determined by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test
Melatonin rescues the intestinal microbial dysbiosis induced by HS in dairy goats
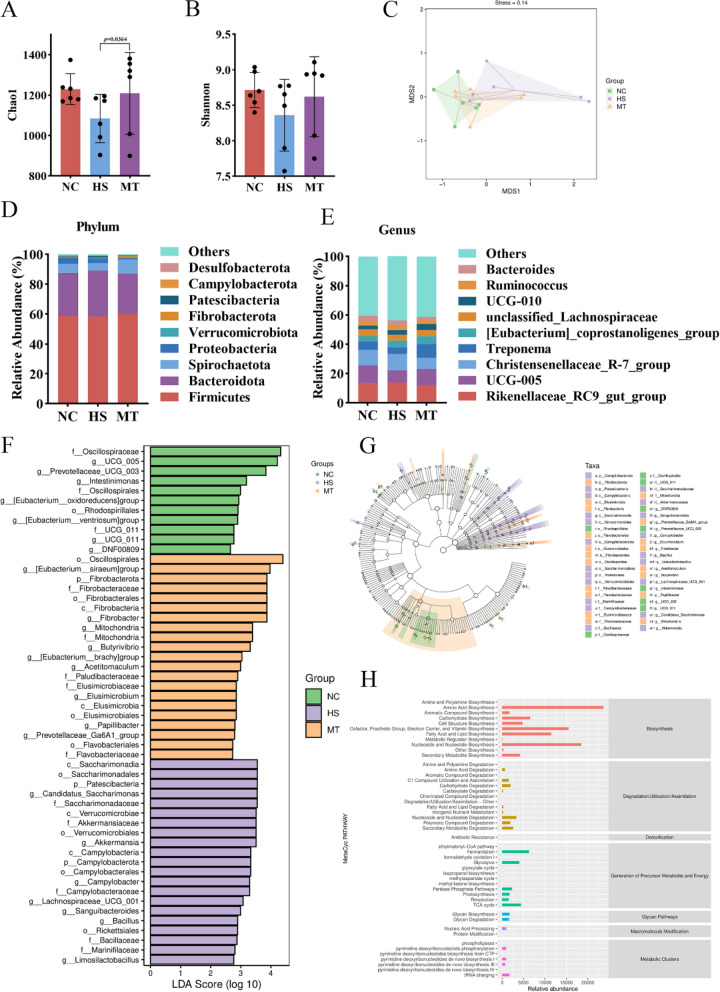
To determine the role of intestinal flora in the protective effect of melatonin against HS-induced reproductive injury in male dairy goats, 16S rRNA high-throughput sequencing technology was employed to assess the impact of melatonin on the fecal microflora of dairy goats under HS. Based on α-diversity analysis using chao1 and Shannon indices, the HS group exhibited a decreasing trend in both indices compared to the NC group, indicating a reduction in the richness and diversity of the intestinal microbiota. Conversely, the MT group showed a significant increase in the chao1 index (P < 0.05) compared to the HS group, suggesting a marked improvement in the richness of intestinal microbiota (Fig. 3A, B). NMDS analysis showed that the intestinal flora clustering of dairy goats in the HS group was significantly different from that in the NC group, and the intestinal flora structure in the MT group displayed a regression trend to the NC group, Permutational multivariate analysis of variance (PERMANOVA) by Adonis was used to determine statistical significance (Fig. 3C). The analysis of the intestinal microbial composition of three groups of dairy goats revealed that, at the phylum level, the dominant microbial categories in their feces include Firmicutes, Bacteroidetes, and Spirochaetota, with cumulative relative abundances significantly exceeding 90% (Fig. 3D). However, at the more detailed genus level, significant changes in the abundances of bacterial groups such as Rikenellaceae_RC9_gut_group, UCG-005, Christensenellaceae_R-7_group, and Treponema were observed in the HS group of dairy goats. Encouragingly, the study also found that melatonin can effectively reverse the microbial community changes induced by HS (Fig. 3E). The LEfSe Analysis revealed a total of 53 taxonomic groups with significant differences, ranging from the phylum to the genus level (Fig. 3F, G). Particularly, compared with the HS group, the relative abundance of f_Marinifilaceae, g_Campylobacter, g_Candidatus_Stoquefichus, and g_Bacillus in MT group was decreased, and conversely, the relative abundance of f_Flavobacteriaceae, f_Paludibacteraceae, g_Acetitomaculum, g_Butyrivibrio, g_Fibrobacter, and g_Prevotellaceae_Ga6A1_g was increased (Supplementary Figure S2). Furthermore, bacterial metabolic function predicted by Phylogenetic Investigation of Communities by Reconstruction of Unobserved States 2 (PICRUSt2) showed that differential metabolic pathways were mainly concentrated in biosynthesis (Fig. 3H), including amino acid biosynthesis; nucleoside and nucleotide biosynthesis; cofactor, cogroup, electron carrier, vitamin biosynthesis; fatty acid and lipid biosynthesis, etc. In conclusion, the intestinal microecological balance of dairy goats is destroyed by HS, and melatonin may reduce the influence of HS by reshaping the intestinal flora to regulate metabolites.
Fig. 3.
Melatonin can regulate intestinal flora dysregulation induced by HS in dairy goats. A α diversity Chao1 index. B Shannon index. C NMDS analysis (stress = 0.14, PERMANOVA by Adonis). D The community structure of each group was analyzed at the phylum level. E The community structure of each group was analyzed at the genus level. F Distribution histogram of different bacteria in three groups of dairy goats based on LDA score. G Taxonomic branching map obtained by LEfSe sequence analysis. H Predicted MetaCyc secondary functional pathway abundance map. In A, B P values were determined by one-way analysis of variance (ANOVA) with Dunnett’s multiple comparison test
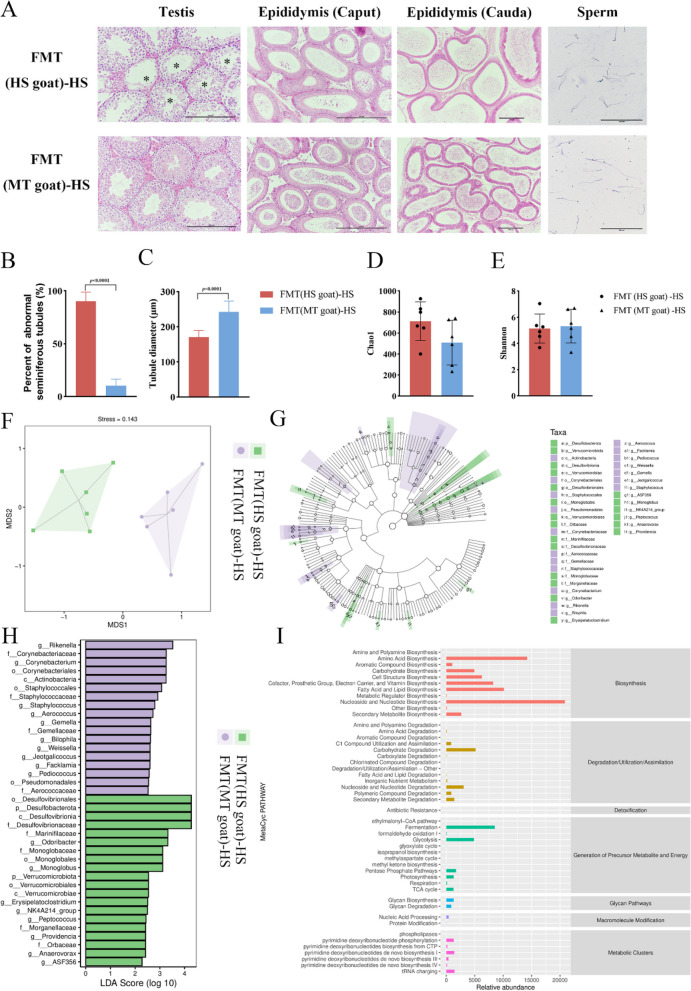
FMT of MT goat reconstruct the protective role of melatonin on sperm quality and testicular in mice
To study whether the protective role of melatonin in testis is through intestinal microbiota, we transplanted rectal fecal microbiota of dairy goats in the HS group and MT group into mice for 2 weeks, followed by 7 days of HS. Before FMT, the mice were treated with a mixture of Abx in the form of drinking water for 1 week, and RT-PCR was used to check the efficiency of Abx in clearing the flora. The majority of the gut-native microbiota was eliminated (Supplementary Figure S3A, B). The histological evaluation of testis and epididymis showed that the testicular tissue structure of mice in the FMT (HS goat) -HS group was damaged, and notably, fecal microbiota from MT goats alleviated the damage of testicular tissue in mice (Fig. 4A), and the results showed that the testicular tissue structure of mice in the FMT (HS goat) -HS group was damaged. The specific manifestations were atrophy of the convoluted seminiferous tubule, separation of testicular cells from the base membrane of the convoluted seminiferous tubule, death, and loss of germ cells. The number of mature sperm in the epididymal tube at the head and tail of the epididymis decreased sharply. The testicular tissue structure of mice receiving MT dairy goat microflora displayed an intact barrier, decreased number of abnormal tubules (P < 0.01), increased diameter of tubules (P < 0.01), integrated basement membrane of tubules, closely arranged cells, and increased number of spermatogenesis cells (Fig. 4B, C). In addition, Giemsa staining showed that the concentration of mature sperm in the FMT (HS goat)-HS group decreased and curled sperm increased after HS, while the morphology of mature sperm in the epididymis of the FMT (HS goat)-HS group was relatively normal. These results suggest that the repair of reproductive damage in FMT (MT goat)-HS mice is at least partially attributable to the gut microbiota of MT dairy goats, and that melatonin can mitigate HS-induced testicular damage and sperm quality reduction in mice by regulating the gut microbiota.
Fig. 4.
FMT modulates the gut microbiota composition of heat-stressed mice. A HE staining of tissue sections from the testis, head of the epididymis, and tail of epididymis in FMT (HS goat) -HS and FMT (MT goat) -HS groups (scale = 250 µm), and giemsa staining of sperm in the tail of epididymis group (scale = 500 µm). The red asterisk represents abnormally curved seminiferous tubules. B The percentage of abnormal curved seminiferous tubules in each group of testicular slices of mice was observed under the microscope in 10 representative fields. C Statistical diagram of spermatogenic tubule diameter. Three sections were taken from each mouse and 149 spermatogenic tubules were used to measure the diameter. D α diversity Chao1 index. E Shannon index. F Analysis of NMDS among FMT mice in each group (stress = 0.143). G Taxonomic branching map obtained by LEfSe sequence analysis. H Histograms of different bacteria distribution in two groups of FMT mice based on LDA scores. I Predicted MetaCyc secondary functional pathway abundance map. In B–E, statistical significance was assessed by independent samples t-test
Microbiota sequencing of FMT mice exhibited no significant difference in α-diversity evaluated by the Chao1 index and Shannon index between the two groups (Fig. 4D, E). Using NMDS analysis, we found that the overall beta-diversity of gut microbial composition was significantly different between the two groups (Fig. 4F). Like the changes of gut microbiota in dairy goats under early HS, the microbiota from Bacteroidetes and Firmicutes phyla was a significant difference between the two groups of FMT mice. At the genus level, compared to the FMT (HS goat)-HS group, the abundance of Limosilactobacillus, Lactobacillus, and unclassified_Lachnospiraceae significantly increased in the FMT (MT goat)-HS group, while the abundance of ligilactobacillus and Lachnospiraceae_NK4A136_group decreased (Supplementary Figure S3C, D). In addition, LEfSe analysis [36] found 38 biomarkers in the two groups, looking for marker species with significant differences between the two groups of FMT mice. In the FMT (HS goat)-HS group of dairy goats, the relative abundance of f_Marinifilaceae has experienced a notable increase. Conversely, the abundance of f_Marinifilaceae in the FMT (MT goat)-HS group of dairy goats has exhibited a distinct downward trend, resulting in a significant reversal. This change aligns with the abundance of f_Marinifilaceae observed in the MT group of dairy goats (Fig. 4G, H, Supplementary Figure S4). Furthermore, consistent with the results of dairy goats, differential metabolic pathways identified by MetaCyc metabolic reference database were also mainly concentrated in amino acid biosynthesis, nucleoside and nucleotide biosynthesis, fatty acid and lipid biosynthesis and other bioanabolic pathways (Fig. 4I). The results showed that FMT could regulate the composition of intestinal flora in heat-stressed mice, and melatonin could regulate testicular tissue metabolism by remodeling intestinal flora, and alleviate the testicular injury and sperm quality decline caused by HS in dairy goats.
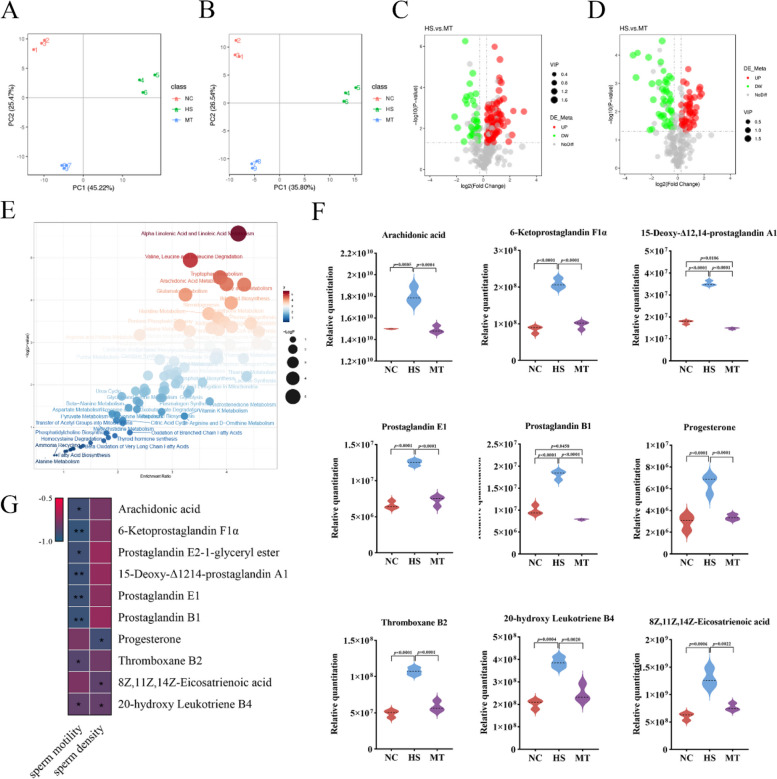
Melatonin attenuates the disorder of metabolites in the testicular of dairy goats under HS
It has been reported that HS can damage the intestinal barrier in animals, and permeability increases with the infiltration of macrophages, eventually leading to the penetration of toxic substances and bacterial compounds [39]. Similarly, our previous research has also revealed that HS-induced necroptosis in the testis is associated with an increase in the level of pro-inflammatory factors in the circulatory system, which is caused by changes in intestinal permeability, microbiota, and metabolism [6]. Here, we conducted non-targeted metabolomics studies on the testicular tissues of dairy goats in the NC group, HS group, and MT group. The results showed that 335 metabolites were identified in positive ion mode and 303 metabolites in negative ion mode. Principal component analysis (PCA) showed that there were significant metabolic differences in testicular tissue between the NC group, the HS group, and the MT group (Fig. 5A, B). We screened differential metabolites based on VIP, FC, and P-value, setting thresholds as VIP > 1.0, FC > 1.2 or FC < 0.833 and P-value < 0.05. Significant differences were observed in 119 types of positive ion mode metabolites between the HS group and the MT group, of which 87 metabolites had increased and 32 had decreased. There were 97 negative ion mode metabolites with significant differences, of which 52 metabolites increased and 45 metabolites decreased (Fig. 5C, D). Moreover, the hierarchical cluster analysis (HCA) of all the diverse metabolites revealed the existence of numerous metabolites displaying comparable abundance patterns in both the NC group and the MT group (Supplementary Figure S5). Quantitative metabolite concentration analysis (qMSEA) showed that the differential metabolites in testis of dairy goats were mainly involved in metabolic pathways such as alpha-linolenic acid and linoleic acid metabolism, valine, leucine, and isoleucine degradation, tryptophan metabolism, AA metabolism (Fig. 5E). To characterize the changes of metabolites in the testis of dairy goats under biological pathways, we conducted a quantitative analysis of metabolite concentrations. The results indicate that under HS conditions, metabolites associated with AA in the testicular tissue of dairy goats exhibited significant alterations. Nevertheless, it is noteworthy that compared to the NC group, the changes in AA-related metabolites in the testicular tissue of melatonin-treated dairy goats were not significant, suggesting that MT exerts a regulatory effect on the changes in testicular metabolites of dairy goats under HS (Fig. 5F, Supplementary Figure S6). Furthermore, through a thorough analysis of the correlation between AA and its related metabolites and the sperm density and motility in three groups of dairy goats, nine metabolites, including 6-Ketoprostaglandin F1α, 15-Deoxy-Δ12,14-prostaglandin A1, Prostaglandin E1, Prostaglandin B1, Progesterone, Thromboxane B2,8Z,11Z,14Z-Eicosatrienoic acid, thromboxane B2, H8: 8Z,11Z, 14Z-eicosatrienoic acid, and 20-hydroxy leukotriene B4 (Fig. 5G), exhibited a highly significant association. Therefore, we speculate that melatonin may regulate AA metabolism by remodeling intestinal flora to alleviate HS-induced testicular injury and sperm quality decline in dairy goats.
Fig. 5.
Melatonin alleviates metabolic disorders of testis induced by HS in dairy goats. A, B PCA of metabolites: positive ion mode (A) negative ion mode (B). C, D Volcanic map of differential metabolites: HS group compared with MT group in positive ion mode (C), HS group compared with MT group in negative ion mode (D). E Quantitative metabolite concentration analysis. F Concentrations of AA-related metabolites (AA, 6-Ketoprostaglandin F1α, 15-Deoxy-Δ12,14-prostaglandin A1, Prostaglandin E1, Prostaglandin B1, Progesterone, Thromboxane B2, 8Z,11Z,14Z-Eicosatrienoic acid, thromboxane B2, H8:8Z,11Z, 14Z-eicosatrienoic Acid, 20-hydroxy leukotriene B4). G Correlation analysis of AA-related metabolites with sperm motility and density. *P < 0.05, **P < 0.01. A one-way analysis of variance (ANOVA) statistical assay was performed to calculate the variations within the detected metabolites, P-values had been corrected for multiple hypothesis testing
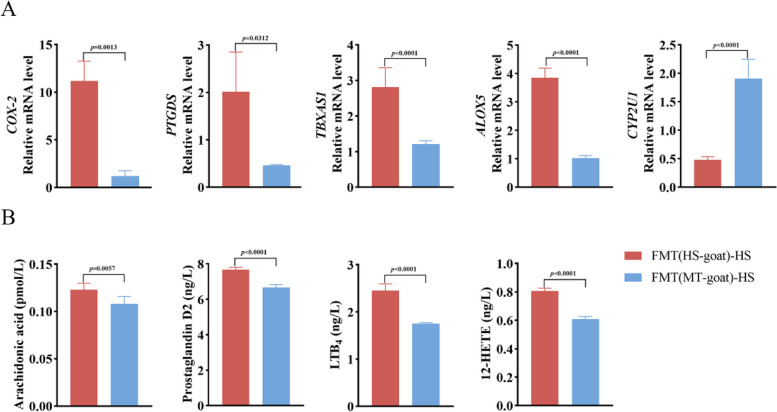
AA is involved in FMT-induced testicular tissue injury and dyszoospermia in HS mice
AA metabolic network is the main network that produces inflammatory mediators and induces inflammation. AA forms hundreds of bioactive metabolites catalyzed by the action of cycoperoxidase, lipoxygenase, and cytochrome oxidase. To substantiate the hypothesis that melatonin exerts regulatory effects on AA metabolism within testicular tissue by modulating the gut microbiota, we detected the levels of AA, AA metabolites, and AA metabolism-related enzymes in the testicular tissue of two groups of FMT mice. The results showed that the expression of COX-2 (P < 0.01), PTGDS (P < 0.05), ALOX5 (P < 0.01), and TBXAS1 (P < 0.01) enzymes in testicular tissue of FMT (HS goat) -HS group was significantly increased, while CYP2U1 (P < 0.01) enzyme was considerably decreased (Fig. 6A). The levels of AA (P < 0.01), PGD2 (P < 0.01), LTB4 (P < 0.01), and 12-HETE (P < 0.01) were significantly increased (Fig. 6B), suggesting that AA metabolism was disturbed in testis of FMT (HS goat) -HS group. However, AA metabolites and related enzyme indexes of mice in the FMT (MT goat) -HS group tended to be normal (Fig. 6A, B), indicating that intestinal flora of dairy goats in the MT group could improve the abnormal metabolism of AA in HS mice. The expression of COX-2, PTGDS, ALOX5, TBXAS1, CYP2U1, and other enzymes in the testis of HS mice was reversed. Therefore, we proposed that melatonin might regulate AA metabolism by remodeling intestinal flora to alleviate HS-induced testicular injury and sperm quality decline in dairy goats.
Fig. 6.
FMT can regulate AA metabolism disorder of testicular tissue in heat-stressed mice. A Expression levels of COX-2, PTGDS, TBXAS1, ALOX5, and CYP2U1 in testicular tissue of mice in FMT (HS goat) -HS and FMT (MT goat) -HS groups. B Concentration of AA, prostaglandin D2, leukotriene B4, and 12-HETE in testis tissue of mice in FMT (HS goat) -HS and FMT (MT goat) -HS groups. In A, B, statistical significance was assessed by independent samples t-test
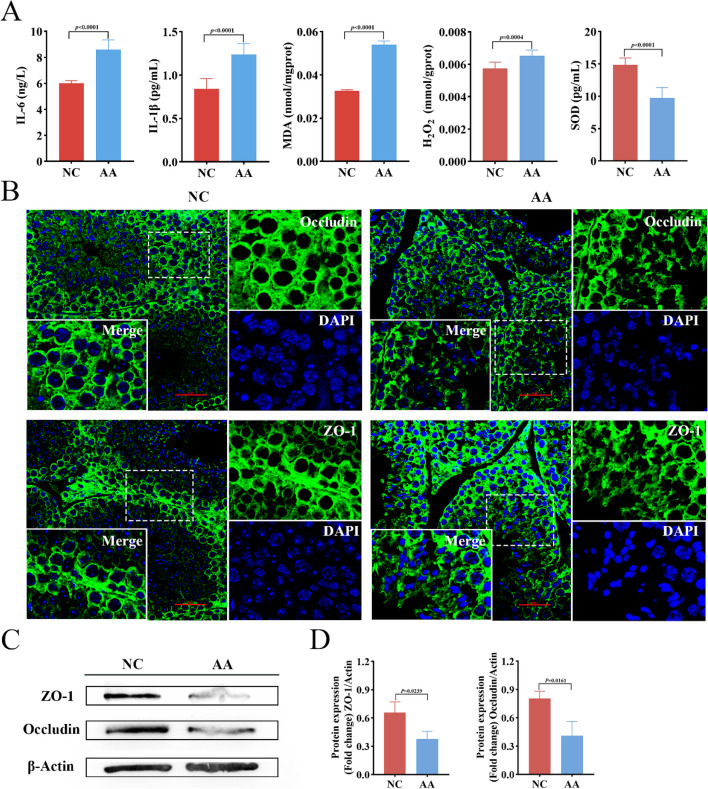
AA supplementation caused spermatogenic dysfunction in male mice
To further investigate the direct metabolic role of AA within the body, we conducted a 28-day AA gavage treatment specifically on mice. The results showed that compared with the NC group, the levels of AA (P < 0.01), PGD2 (P < 0.01), LTB4 (P < 0.01), and 12-HETE (P < 0.01) in the testicular tissue of the AA group were significantly increased (Fig. 7A), indicating that AA metabolism disorders occurred in the testis of mice in the AA group. The H&E staining results of the testicular and epididymal tissues revealed that AA significantly caused testicular damage, manifesting as disrupted spermatogenic cells, even loss of these cells, atrophy of seminiferous tubules (P < 0.01), and a notable increase in the number of abnormal seminiferous tubules (P < 0.01) (Fig. 7B, D). We then proceeded to assess whether AA caused inflammation and elevated levels of oxidative stress in mouse testicular tissue. Compared to the NC group, the levels of IL-6 (P < 0.01) and IL-1β (P < 0.01) in the testicular tissue of mice in the AA group exhibited a notable upward trend. Concurrently, the oxidative stress-related indicators, H2O2 (P < 0.01) and MDA (P < 0.01), also increased significantly, while the content of the antioxidant enzyme SOD (P < 0.01) decreased significantly. These results indicate that there exists a mutually promotional relationship between oxidative stress and inflammatory responses in the testicular tissue of mice in the AA group, and this interaction further exacerbates the damage to the testicular tissue (Fig. 8A). These results are consistent with those previously observed in HS dairy goats, further confirming that AA treatment can cause damage to the male reproductive function. The damaging effect of AA on the integrity of the mouse testicular cell barrier was evaluated by immunofluorescence and western blot. The AA group showed severe cell membrane disruption and disorder, indicating that AA impinges on the development and function of cells in the testis (Fig. 8B). Western blot results analysis that compared with the NC group, the expression of specific tight junction proteins occludin (P < 0.05) and ZO-1 (P < 0.05) in the testicular tissue of mice in the AA group decreased (Fig. 8C, D). These results showed that AA could disrupt the tight connections between testicular cells, leading to spermatogenesis disorders in mice.
Fig. 8.
AA causes testicular inflammation and oxidative stress, and destroys the intercellular barrier in mice. A The content of IL-6, IL-1β, MDA, H2O2, and SOD in the testis tissue of mice in the NC group and AA group was detected by ELISA. B Immunofluorescence images of occludin and ZO-1 in testicular tissue in different groups. Scale bars = 20 μm. (Each dashed white box represents the enlarged position in the merge graph in the lower left corner). C, D Western blotting showing TJPs (occludin and ZO-1) expression in different groups; densitometric data were normalized to actin (n = 3). In A, D, statistical significance was assessed by independent samples t-test
Discussion
Heat stress (HS), a pervasive stressor across multiple tissues, has been extensively studied in its impact on animal health. Specially, it was demonstrated that HS has adverse effects on male reproductive damage, particularly, the decrease of spermatogenesis and intestinal functions in dairy goats [1, 6]. Consistently, the present study in a comprehensive phenotypic assessment of HS-induced male reproductive injury in dairy goats revealed a notable decline in sperm motility and an elevated rate of sperm malformation through the substantial increase of abnormal tubules in testicular tissue. Melatonin, a neuroendocrine hormone renowned for its potent broad-spectrum antioxidant properties, is effectively employed as an exogenous supplement to alleviate reproductive injuries [40, 41]. While the role of melatonin has been studied, how it acts remains unclear. In this study, we explored the role of melatonin in reproduction injury caused by HS in dairy goats and particularly its potential mechanism. We found that melatonin limits the excessive release of AA and destruction of the intercellular barrier integrity in the testis through reshaping gut microbiota, thereby protecting dairy goats from reproduction injury caused by HS.
HS is demonstrated to reduce semen quality spermatogenesis by damaging Sertoli cells, breaking the BTB, and decreasing the population of germ cells [2, 7, 42, 43]. Recent scientific evidence suggests a correlation between microbiome dysregulation and reproductive disorders or diseases [10, 44–46]. The present study revealed that HS male dairy goats with reproductive dysfunction were accompanied by disturbance of gut microbiota, and FMT of HS male dairy goats aggravated spermatogenesis disorder of mice, indicating a positive relationship between microbial modification and aggravated spermatogenesis disorder. Consistently, the gut microbiota is demonstrated to be an integral component of the host and has a crucial impact on host health [47]. Melatonin was applied to prevent HS-induced reproductive impairment. Investigators have verified that the interaction mechanism between melatonin and intestinal microbiota constitutes a complex and multifaceted network. Melatonin has been shown to be able to regulate the balance of intestinal microbiota in obese mice, such as reducing the ratio of Firmicutes to Bacteroidetes and decreasing the abundance of mucin-degrading bacteria Akkermansia, which is associated with healthy mucosal tissues [25]. Whether exogenous melatonin prevents HS-induced reproductive impairment by reshaping the gut microbiota is still unsubstantiated. Here, we found that the gut microbiota from HS dairy goats pretreated with melatonin was changed, particularly decreased abundance of inflammation-linked bacteria, such as f_Marinifilaceae, g_Campylobacter, g_Candidatus_Stoquefichus, and g_Bacillus. The g_Campylobacter serves as a typical bacterium that typically causes enteritis, and g_Candidatus_Stoquefichus is strongly associated with intestinal and skin inflammation [48]. Therefore, the presence of these bacteria might lead to more severe infections and intestinal dysfunction, thereby indicating an elevated risk of disease occurrence [48–51]. In line, melatonin not only significantly reduces the population of pathogenic microbiota but also increases the relative abundance of bacteria such as g_Acetitomaculum, g_Butyrivibrio, bacteria involved in cellulose digestion (g_Fibrobacter), and short-chain fatty acid-producing bacteria (g_Prevotellaceae_Ga6A1_group) [52, 53]. Interestingly, the mice transplanted with fecal bacteria from HS dairy goats exhibited more serious testicular damage and spermatogenic dysfunction. Inversely, the mice with fecal microbiota transfer of MT dairy goats displayed a recovery from reproductive injury, suggesting that gut microbiota plays a crucial role in mediating the protective effect of melatonin against reproductive injury induced by HS. These findings further reinforced the hypothesis that melatonin’s protective effect on male reproductive health is attained through the regulation of microbial ecological equilibrium. Notably, like the changes observed in dairy goats, there were significant alterations in the abundance of Bacteroidetes and Firmicutes in the guts of recipient mice. Consistently, melatonin has been shown to be able to regulate the balance of intestinal microbiota in obese mice, such as reducing the ratio of Firmicutes to Bacteroidetes and decreasing the abundance of mucin-degrading bacteria Akkermansia, which is associated with healthy mucosal tissues. These findings further reinforced the hypothesis that melatonin’s protective effect on male reproductive health is attained through the regulation of microbial ecological equilibrium. Despite the importance of gut microbiota in maintaining reproductive health and providing valuable insights into potential therapeutic strategies for male reproductive disorders, the bacteria involved in protection are still unclear.
Besides the direct effects of gut microbiota, metabolites of gut microbiota could also impact body health. In the present study, the metabolic pathways in the testicular tissue of dairy goats were primarily affected by changes in alpha-linolenic acid and linoleic acid metabolism, as well as AA metabolism. AA, a key polyunsaturated fatty acid (PUFA), is primarily derived from linoleic and linolenic acids in mammalian metabolism, which plays a significant role in various cellular physiological processes, such as inflammation and immunity [54]. Unsaturated fatty acids are converted into important regulatory substances like prostaglandins and leukotrienes, which participate in the systemic regulation of bodily functions [55]. We speculated that AA mediated by gut microbiota was also associated with worsened male reproductive damage. Unsurprisingly, this study revealed that the levels of AA and its metabolites in the testicular tissue of HS dairy goats were significantly higher compared to those in normal testicular tissue [54]. Increasing evidence proved that the conversion of AA to isoprostaglandins mediated by intestinal microbiota can be detected in biological fluids or sperm membranes, serving as specific markers of exposure to chemical agents, oxidative damage, compromised antioxidant response, and sperm immaturity [54, 56]. This study conducted a thorough analysis of the effects of AA on the testicular tissue of mice. The results showed that, compared to the NC group, mice in the AA group exhibited a significant increase in the levels of IL-6 and IL-1β in their testicular tissue. Simultaneously, there was a notable upward trend in the content of MDA, while the activity of SOD was significantly reduced. These findings strongly suggested that AA can trigger inflammatory responses and oxidative stress, leading to the exacerbation of lipid peroxidation. Of particular concern, AA also disrupted the intercellular connections within the testes, resulting in severe damage to the integrity of the cellular barrier. Ultimately, this damage impaired spermatogenesis in male mice, exerting significant adverse effects on the function and development of testicular cells. Future research must delve deeper into how prostaglandins and other eicosanoids influence male reproductive processes. This effort may include exploring the effects of these molecular-level changes on sperm production, motility, and morphology, as well as their potential involvement in inflammatory processes within the male reproductive system. Moreover, when it comes to practical applications, melatonin's effectiveness exhibits species-specific differences, lacking a consensus on the optimal dosage, timing, and method of administration. Our study, based on the gut microbiome-testicular axis, elucidated that melatonin alleviates HS-induced spermatogenesis disorders in male dairy goats by reshaping the gut microbiota and ameliorating its induced arachidonic acid metabolism disorders. On this basis, we further explored the optimal dosage and administration method of melatonin sustained-release agent in the actual production environment to ensure its effectiveness and safety in practical applications. This study provided crucial theoretical support and practical guidance for enhancing the reproductive performance of male dairy goats in high summer temperatures. Additionally, it offered effective and reliable targets for the prevention and treatment of climate-induced infertility in male livestock, presenting novel ideas and methodologies for related prevention and control efforts.
Conclusion
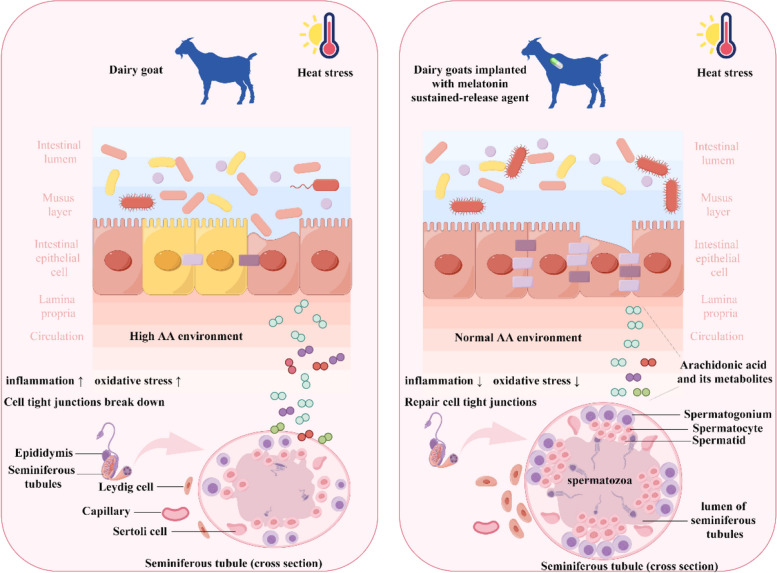
In conclusion, this study’s findings highlight the protective role of melatonin against reproductive injury in HS male dairy goats. The mechanistic study reveals that melatonin effectively suppresses excessive AA synthesis in the testis tissue by modulating the intestinal microbiota. This modulation plays a pivotal role in mitigating oxidative stress and dampening inflammatory responses in the testis tissue of dairy goats, and restoring intercellular connections within testicular cells (Fig. 9).
Fig. 9.
Overview schematic of the study. Heat stress disturbs intestinal flora homeostasis and causes dysregulation of AA metabolism in testicular tissue, thus leading to male reproductive injury in dairy goats. Melatonin can reconstruct intestinal flora balance to repair the abnormal metabolism of AA, reduce oxidative stress and inflammatory response in testicular tissue, and repair the connection between testicular cells, thus alleviating the male reproductive injury induced by HS in dairy goats
Supplementary Information
Additional file 1: Figure S1. Testis morphology of dairy goats in NC group, HS group and MT group. The red arrow points to the epididymis. Figure S2. Microflora abundance histogram of different groups in dairy goats. The distinct bacterial groups on the left, from top to bottom, are f__Marinifilaceae, g__Campylobacter,g__Candidatus_Stoquefichus and g__Bacillus. The different bacterial groups on the right side, from top to bottom, are f__Flavobacteriaceae,g__paludibacteraceae,g__Acetitomaculum,g__Butyrivibrio, g__Fibrobacter,g__Prevotellaceae_Ga6A1_group. Figure S3. Fecal microbiota transplantation validation test. A-B Antibiotics reduced the number of gut bacteria in the mice. (A) The bacterial universal 16S rRNA gene (V3-V4 region) was amplified by q-PCR and the total bacterial population was quantified. (B) Mice were intragastric with a combination of antibiotics for 7 days, eliminating most of the natural gut microbiota (n=10 per group). Data are represented as mean ± SD. Statistical significance was assessed by independent samples t-test. C The community structure of each group was analyzed at the phylum level. D The community structure of each group was analyzed at the genus level. Figure S4. Histograms of flora abundance in different groups of fecal bacteria transplanted mice. In the figure, FMT (HS goat) -HS group and FMT (MT goat) -HS group are labeled as FMT__HS and FMT__MT, respectively. The distinct bacterial groups on the left, from top to bottom, are f__Marinifilaceae,g__Erysipelatoclostridium, g__Monoglobus, g__ASF356, g__Peptococcus,g__Anaerovorax, g__NK4A214_group, g__Orbaceae, g__Providenciaand g__Desulfovibrionales. The different bacterial groups on the right side, from top to bottom, are g__Rikenella, g__Facklamia, g__Aerococcus,g__Gemella, g__Weissella, g__Staphylococcus, g__Pediococcus,g__Corynebacterium and g__Bilophila. Figure S5. Differential metabolite clustering heat maps of testicular tissue of dairy goats in NC group, HS group and MT group. A Positive ion pattern differential metabolite cluster heat map. B negative ion pattern differential metabolite cluster heat map. The vertical cluster is the sample cluster, the horizontal cluster is the metabolite cluster, and the shorter the cluster branch, the higher the similarity. The relationship of metabolite content clustering among groups can be seen through horizontal comparison. Figure S6. The most enriched pathway terms of HS.vs.MT_neg. Pathview analysis of Arachidonic acid metabolism KEGG pathway. Supplementary Table S1. THII index. Supplementary Table S2. Primers information Supplementary Table S3. Antibodies information.
Acknowledgements
The authors would like to thank all members of Shaanxi Aonike dairy goat breeding Co., LTD for assisting us in conducting experiments related to dairy goats.
Abbreviations
- AA
Arachidonic acid
- LPS
Lipopolysaccharide
- THI
Temperature and humidity index
- Abx
Antibiotic cocktail
- PGD2
Prostaglandin D2
- LTB4
Leukotriene B4
- 12-HETE
12-Hydroxyeicosane tetraenoic acid
- LC-MS
Liquid chromatograph mass spectrometer
- LDA
Linear discriminant analysis
- LEfSe
Linear discriminant analysis effect size
- PCA
Principal component analysis
Authors’ contributions
SP, BHM and XRG participated in the study design; SP gets funding; XRG, JX, TSF, MW, YL, HW were tested. YKZ and JW analyzed the data; YL, CLW, XQW and HYH conducted data management, methodological data visualization; XRG and YZW made manuscript edits; SP and XRG wrote the manuscript; SP and BHM are responsible for conceptualization, monitoring and funding; Mathias L. RICHARD, Harry SOKOL, SP, BHM and YZW conducted manuscript review. All authors have read and approved the manuscript.
Funding
This study was supported by the National Natural Science Foundation of China (32072815), Grants from the National Key Research and Development Program of China (2022YFD1300200), the General Project of the Key R & D Plan of Shaanxi Province (2023-YBNY-140) and Horizontal Research Funding from Ningbo Second Hormone Factory (TG20221184).
Data availability
This study of 16s rRNA original sequencing data have been deposited in NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/) under the accession numbers PRJNA1087075 and PRJNA1087076; This study of targeted metabolomic raw sequence data have been deposited in the MetaboLights database (https://www.ebi.ac.uk/metabolights/), submit for MTBLS9764.
Declarations
Ethics approval and consent to participate
All experimental animals and procedures were performed in strict accordance with the Guide for the Care and Use of Laboratory Animals (Ministry of Science and Technology of the People’s Republic of China, Policy No. 2006398) and were approved by the Animal Care and Use Center of Northwest A&F University, Shaanxi, China (approval No. 201902A299).
Consent for publication
Not applicable.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Yazhou Wang, Email: wangyazhou@nwafu.edu.cn.
Baohua Ma, Email: mabh@nwsuaf.edu.cn.
Sha Peng, Email: pengshacxh@nwsuaf.edu.cn.
References
- 1.Liu Y, Cai H, Guo X, Aierken A, Hua J, Ma B, et al. Melatonin alleviates heat stress-induced testicular damage in dairy goats by inhibiting the PI3K/AKT signaling pathway. Stress Biol. 2022;2(1):47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fan X, Xi H, Zhang Z, Liang Y, Li Q, He J. Germ cell apoptosis and expression of Bcl-2 and Bax in porcine testis under normal and heat stress conditions. Acta Histochem. 2017;119(3):198–204. [DOI] [PubMed] [Google Scholar]
- 3.Mieusset R, Bujan L. Testicular heating and its possible contributions to male infertility: a review. Int J Androl. 1995;18(4):169–84. [DOI] [PubMed] [Google Scholar]
- 4.Shahat AM, Rizzoto G, Kastelic JP. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology. 2020;158:84–96. [DOI] [PubMed] [Google Scholar]
- 5.Agarwal A, Said TM. Role of sperm chromatin abnormalities and DNA damage in male infertility. Hum Reprod Update. 2003;9(4):331–45. [DOI] [PubMed] [Google Scholar]
- 6.Cai H, Qin D, Liu Y, Guo X, Liu Y, Ma B, et al. Remodeling of gut microbiota by probiotics alleviated heat stroke-induced necroptosis in male germ cells. Mol Nutr Food Res. 2023;67(18):e2300291. [DOI] [PubMed] [Google Scholar]
- 7.Qin DZ, Cai H, He C, Yang DH, Sun J, He WL, et al. Melatonin relieves heat-induced spermatocyte apoptosis in mouse testes by inhibition of ATF6 and PERK signaling pathways. Zool Res. 2021;42(4):514–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cai H, Cao X, Qin D, Liu Y, Liu Y, Hua J, et al. Gut microbiota supports male reproduction via nutrition, immunity, and signaling. Front Microbiol. 2022;13:977574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ding N, Zhang X, Zhang XD, Jing J, Liu SS, Mu YP, et al. Impairment of spermatogenesis and sperm motility by the high-fat diet-induced dysbiosis of gut microbes. Gut. 2020;69(9):1608–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jin Z, Yang Y, Cao Y, Wen Q, Xi Y, Cheng J, et al. The gut metabolite 3-hydroxyphenylacetic acid rejuvenates spermatogenic dysfunction in aged mice through GPX4-mediated ferroptosis. Microbiome. 2023;11(1):212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reiter RJ, Tan DX, Burkhardt S. Reactive oxygen and nitrogen species and cellular and organismal decline: amelioration with melatonin. Mech Ageing Dev. 2002;123(8):1007–19. [DOI] [PubMed] [Google Scholar]
- 12.Tan DX, Manchester LC, Terron MP, Flores LJ, Reiter RJ. One molecule, many derivatives: a never-ending interaction of melatonin with reactive oxygen and nitrogen species? J Pineal Res. 2007;42(1):28–42. [DOI] [PubMed] [Google Scholar]
- 13.Vaghari-Tabari M, Moein S, Alipourian A, Qujeq D, Malakoti F, Alemi F, et al. Melatonin and inflammatory bowel disease: from basic mechanisms to clinical application. Biochimie. 2023;209:20–36. [DOI] [PubMed] [Google Scholar]
- 14.de Faria PJ, Feltes BC, Bonatto D. Melatonin as a central molecule connecting neural development and calcium signaling. Funct Integr Genomics. 2011;11(3):383–8. [DOI] [PubMed] [Google Scholar]
- 15.Mazzoccoli G, Carughi S, Sperandeo M, Pazienza V, Giuliani F, Tarquini R. Neuro-endocrine correlations of hypothalamic-pituitary-thyroid axis in healthy humans. J Biol Regul Homeost Agents. 2011;25(2):249–57. [PubMed] [Google Scholar]
- 16.Xu LX, Lv Y, Li YH, Ding X, Wang Y, Han X, et al. Melatonin alleviates brain and peripheral tissue edema in a neonatal rat model of hypoxic-ischemic brain damage: the involvement of edema related proteins. BMC Pediatr. 2017;17(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chang CC, Huang TY, Chen HY, Huang TC, Lin LC, Chang YJ, et al. Protective effect of melatonin against oxidative stress-induced apoptosis and enhanced autophagy in human retinal pigment epithelium cells. Oxid Med Cell Longev. 2018;2018:9015765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Onaolapo OJ, Onaolapo AY, Olowe OA, Udoh MO, Udoh DO, Nathaniel TI. Melatonin and melatonergic influence on neuronal transcription factors: implications for the development of novel therapies for neurodegenerative disorders. Curr Neuropharmacol. 2020;18(7):563–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vasey C, Mcbride J, Penta K. Circadian Rhythm Dysregulation and Restoration: The Role of Melatonin. Nutrients. 2021;13(10):3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calvo JR, Gonzalez-Yanes C, Maldonado MD. The role of melatonin in the cells of the innate immunity: a review. J Pineal Res. 2013;55(2):103–20. [DOI] [PubMed] [Google Scholar]
- 21.Ren W, Liu G, Chen S, Yin J, Wang J, Tan B, et al. Melatonin signaling in T cells: Functions and applications. J Pineal Res. 2017;62(3):e12394. [DOI] [PubMed] [Google Scholar]
- 22.Liu G, Jiang Q, Chen S, Fang J, Ren W, Yin J, et al. Melatonin alters amino acid metabolism and inflammatory responses in colitis mice. Amino Acids. 2017;49(12):2065–71. [DOI] [PubMed] [Google Scholar]
- 23.Agil A, Navarro-Alarcon M, Ruiz R, Abuhamadah S, El-Mir MY, Vazquez GF. Beneficial effects of melatonin on obesity and lipid profile in young Zucker diabetic fatty rats. J Pineal Res. 2011;50(2):207–12. [DOI] [PubMed] [Google Scholar]
- 24.Chen X, Zhang C, Zhao M, Shi CE, Zhu RM, Wang H, et al. Melatonin alleviates lipopolysaccharide-induced hepatic SREBP-1c activation and lipid accumulation in mice. J Pineal Res. 2011;51(4):416–25. [DOI] [PubMed] [Google Scholar]
- 25.Xu P, Wang J, Hong F, Wang S, Jin X, Xue T, et al. Melatonin prevents obesity through modulation of gut microbiota in mice. J Pineal Res. 2017;62(4):e12399. [DOI] [PubMed] [Google Scholar]
- 26.Kozirog M, Poliwczak AR, Duchnowicz P, Koter-Michalak M, Sikora J, Broncel M. Melatonin treatment improves blood pressure, lipid profile, and parameters of oxidative stress in patients with metabolic syndrome. J Pineal Res. 2011;50(3):261–6. [DOI] [PubMed] [Google Scholar]
- 27.Yin J, Li Y, Han H, Chen S, Gao J, Liu G, et al. Melatonin reprogramming of gut microbiota improves lipid dysmetabolism in high-fat diet-fed mice. J Pineal Res. 2018;65(4):e12524. [DOI] [PubMed] [Google Scholar]
- 28.Wang X, Wang Z, Cao J, Dong Y, Chen Y. Gut microbiota-derived metabolites mediate the neuroprotective effect of melatonin in cognitive impairment induced by sleep deprivation. Microbiome. 2023;11(1):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ren W, Wang P, Yan J, Liu G, Zeng B, Hussain T, et al. Melatonin alleviates weanling stress in mice: Involvement of intestinal microbiota. J Pineal Res. 2018;64(2):e12448. [DOI] [PubMed] [Google Scholar]
- 30.Kleemann DO, Kelly JM, Arney LJ, Tilbrook AJ, Walker SK. Melatonin dose: testicular and testosterone response in border leicester rams during spring. Livest Sci. 2022;260:104928. [Google Scholar]
- 31.Mahjoubi E, Yazdi MH, Aghaziarati N, Noori GR, Afsarian O, Baumgard LH. The effect of cyclical and severe heat stress on growth performance and metabolism in Afshari lambs. J Anim Sci. 2015;93(4):1632–40. [DOI] [PubMed] [Google Scholar]
- 32.Buffington DE, Collazo-Arocho A, Canton GH, Pitt D. Black Globe-Humidity Index (BGHI) as comfort equation for dairy cows. Trans ASABE. 1981;24:711–0714. [Google Scholar]
- 33.Li D, Feng Y, Tian M, Ji J, Hu X, Chen F. Gut microbiota-derived inosine from dietary barley leaf supplementation attenuates colitis through PPARgamma signaling activation. Microbiome. 2021;9(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu L, Liu Y, Cui J, Liu H, Liu YB, Qiao WL, et al. Oxidative stress induces gastric submucosal arteriolar dysfunction in the elderly. World J Gastroenterol. 2013;19(48):9439–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bokulich NA, Kaehler BD, Rideout JR, Dillon M, Bolyen E, Knight R, et al. Optimizing taxonomic classification of marker-gene amplicon sequences with QIIME 2’s q2-feature-classifier plugin. Microbiome. 2018;6(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Segata N, Izard J, Waldron L, Gevers D, Miropolsky L, Garrett WS, et al. Metagenomic biomarker discovery and explanation. Genome Biol. 2011;12(6):R60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wen B, Mei Z, Zeng C, Liu S. metaX: a flexible and comprehensive software for processing metabolomics data. BMC Bioinformatics. 2017;18(1):183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rao G, Sui J, Zhang J. Metabolomics reveals significant variations in metabolites and correlations regarding the maturation of walnuts (Juglans regia L). Biol Open. 2016;5(6):829–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Koch F, Thom U, Albrecht E, Weikard R, Nolte W, Kuhla B, et al. Heat stress directly impairs gut integrity and recruits distinct immune cell populations into the bovine intestine. Proc Natl Acad Sci U S A. 2019;116(21):10333–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ramadan TA, Taha TA, Samak MA, Hassan A. Effectiveness of exposure to longday followed by melatonin treatment on semen characteristics of Damascus male goats during breeding and non-breeding seasons. Theriogenology. 2009;71(3):458–68. [DOI] [PubMed] [Google Scholar]
- 41.Ramadan TA, Kumar D, Ghuman SS, Singh I. Melatonin-improved buffalo semen quality during nonbreeding season under tropical condition. Domest Anim Endocrinol. 2019;68:119–25. [DOI] [PubMed] [Google Scholar]
- 42.Deng CC, Zhang JP, Huo YN, Xue HY, Wang W, Zhang JJ, et al. Melatonin alleviates the heat stress-induced impairment of Sertoli cells by reprogramming glucose metabolism. J Pineal Res. 2022;73(3):e12819. [DOI] [PubMed] [Google Scholar]
- 43.Hu Y, Hu H, Yin L, Wang L, Luo K, Luo N. Arachidonic acid impairs the function of the blood-testis barrier via triggering mitochondrial complex-ROS-P38 MAPK axis in hyperthermal Sertoli cells. Ecotoxicol Environ Saf. 2023;252:114598. [DOI] [PubMed] [Google Scholar]
- 44.Zhang T, Sun P, Geng Q, Fan H, Gong Y, Hu Y, et al. Disrupted spermatogenesis in a metabolic syndrome model: the role of vitamin A metabolism in the gut-testis axis. Gut. 2022;71(1):78–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li A, Li F, Song W, Lei ZL, Sha QQ, Liu SY, et al. Gut microbiota-bile acid-vitamin D axis plays an important role in determining oocyte quality and embryonic development. Clin Transl Med. 2023;13(10):e1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Qi X, Yun C, Sun L, Xia J, Wu Q, Wang Y, et al. Gut microbiota-bile acid-interleukin-22 axis orchestrates polycystic ovary syndrome. Nat Med. 2019;25(8):1225–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krautkramer KA, Fan J, Backhed F. Gut microbial metabolites as multi-kingdom intermediates. Nat Rev Microbiol. 2021;19(2):77–94. [DOI] [PubMed] [Google Scholar]
- 48.Fan X, Zang T, Dai J, Wu N, Hope C, Bai J, et al. The associations of maternal and children’s gut microbiota with the development of atopic dermatitis for children aged 2 years. Front Immunol. 2022;13:1038876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jalanka J, Gunn D, Singh G, Krishnasamy S, Lingaya M, Crispie F, et al. Postinfective bowel dysfunction following Campylobacter enteritis is characterised by reduced microbiota diversity and impaired microbiota recovery. Gut. 2023;72(3):451–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao Y, Feng T, Wu Y, Xu Y, Du L, Wang T, et al. The multi-kingdom microbiome of the goat gastrointestinal tract. Microbiome. 2023;11(1):219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Molina-Santiago C, Vela-Corcia D, Petras D, Diaz-Martinez L, Perez-Lorente AI, Sopena-Torres S, et al. Chemical interplay and complementary adaptative strategies toggle bacterial antagonism and co-existence. Cell Rep. 2021;36(4):109449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sun Z, Yu Z, Wang B. Perilla frutescens leaf alters the rumen microbial community of lactating dairy cows. Microorganisms. 2019;7(11):562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu D, Feng M, Chu Y, Wang S, Shete V, Tuohy KM, et al. The prebiotic effects of oats on blood lipids, gut microbiota, and short-chain fatty acids in mildly hypercholesterolemic subjects compared with rice: a randomized, controlled trial. Front Immunol. 2021;12:787797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu C, Gu L, Hu L, Jiang C, Li Q, Sun L, et al. FADS1-arachidonic acid axis enhances arachidonic acid metabolism by altering intestinal microecology in colorectal cancer. Nat Commun. 2023;14(1):2042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S-1519S. [DOI] [PubMed] [Google Scholar]
- 56.Signorini C, Moretti E, Collodel G. Role of isoprostanes in human male infertility. Syst Biol Reprod Med. 2020;66(5):291–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional file 1: Figure S1. Testis morphology of dairy goats in NC group, HS group and MT group. The red arrow points to the epididymis. Figure S2. Microflora abundance histogram of different groups in dairy goats. The distinct bacterial groups on the left, from top to bottom, are f__Marinifilaceae, g__Campylobacter,g__Candidatus_Stoquefichus and g__Bacillus. The different bacterial groups on the right side, from top to bottom, are f__Flavobacteriaceae,g__paludibacteraceae,g__Acetitomaculum,g__Butyrivibrio, g__Fibrobacter,g__Prevotellaceae_Ga6A1_group. Figure S3. Fecal microbiota transplantation validation test. A-B Antibiotics reduced the number of gut bacteria in the mice. (A) The bacterial universal 16S rRNA gene (V3-V4 region) was amplified by q-PCR and the total bacterial population was quantified. (B) Mice were intragastric with a combination of antibiotics for 7 days, eliminating most of the natural gut microbiota (n=10 per group). Data are represented as mean ± SD. Statistical significance was assessed by independent samples t-test. C The community structure of each group was analyzed at the phylum level. D The community structure of each group was analyzed at the genus level. Figure S4. Histograms of flora abundance in different groups of fecal bacteria transplanted mice. In the figure, FMT (HS goat) -HS group and FMT (MT goat) -HS group are labeled as FMT__HS and FMT__MT, respectively. The distinct bacterial groups on the left, from top to bottom, are f__Marinifilaceae,g__Erysipelatoclostridium, g__Monoglobus, g__ASF356, g__Peptococcus,g__Anaerovorax, g__NK4A214_group, g__Orbaceae, g__Providenciaand g__Desulfovibrionales. The different bacterial groups on the right side, from top to bottom, are g__Rikenella, g__Facklamia, g__Aerococcus,g__Gemella, g__Weissella, g__Staphylococcus, g__Pediococcus,g__Corynebacterium and g__Bilophila. Figure S5. Differential metabolite clustering heat maps of testicular tissue of dairy goats in NC group, HS group and MT group. A Positive ion pattern differential metabolite cluster heat map. B negative ion pattern differential metabolite cluster heat map. The vertical cluster is the sample cluster, the horizontal cluster is the metabolite cluster, and the shorter the cluster branch, the higher the similarity. The relationship of metabolite content clustering among groups can be seen through horizontal comparison. Figure S6. The most enriched pathway terms of HS.vs.MT_neg. Pathview analysis of Arachidonic acid metabolism KEGG pathway. Supplementary Table S1. THII index. Supplementary Table S2. Primers information Supplementary Table S3. Antibodies information.
Data Availability Statement
This study of 16s rRNA original sequencing data have been deposited in NCBI Sequence Read Archive (https://www.ncbi.nlm.nih.gov/) under the accession numbers PRJNA1087075 and PRJNA1087076; This study of targeted metabolomic raw sequence data have been deposited in the MetaboLights database (https://www.ebi.ac.uk/metabolights/), submit for MTBLS9764.