Abstract
Study Design
Retrospective Cohort Study.
Objective
Reconstruction of maxillary bone defects can be completed with vascularized and non-vascularized autografts. Cellular bone matrix allografts (CBMs), which have lineage committed bone cells, has risen as an alternative. The purpose of this study was to describe our experience and to determine the success of CBM based maxillary reconstruction in a variety of clinical scenarios.
Methods
A retrospective cohort study was designed and implemented using data from subjects who presented to the University of Louisville and were treated with a CBM for maxillary reconstruction from 2019 to 2023. Subjects were excluded if they were not treated with a CBM, data were not complete, or postoperative follow-up time was less than 3 months. Descriptive statistics were calculated for each variable. To measure the associations between the risk factors and graft success, Fisher’s exact test was implemented. A P-value of <0.05 was considered significant.
Results
The sample included 48 subjects. The mean age of all subjects was 43 ± 24 years. Overall, 42 (87.5%) cases were successful. The perioperative antibiotic administered (P = 0.02), etiology (P = 0.021), and the addition of platelet rich fibrin or autograft as an adjunct influenced CBM success (P = 0.039).
Conclusions
CBMs are a viable option for reconstruction of maxillary bone defects. CBMs may be an alternative to autografts.
Keywords: maxilla reconstruction, tissue engineering, cellular bone matrix
Introduction
Defects of the maxilla can be the result of developmental anomalies, trauma, infection, or pathology. The midface is anatomically complex, consisting of 15 bones, and a variety of specialized components such as the oral cavity, sinuses, and nasolacrimal system. The tissues removed or missing guide the reconstructive approach; however, truly recapitulating form and function is complex and challenging. Surgical approaches for reconstruction of the maxilla can consist of local and regional flaps, non-vascularized bone grafts, and vascularized tissue transfer. In addition, non-surgical approaches such as obturation are also an option. Recently, tissue engineering and allogenic grafts have risen as an alternative for boney reconstruction of the maxilla.
Tissue engineering is the use of a scaffold, cells, and bioactive molecules, such as growth factors, to promote regeneration of a target tissue. Specifically, in this case, bone. One major benefit of using tissue engineering techniques and allogenic components is a possible decrease in operative time and the need of a second surgical site. Traditionally, only autografts have had all the components to be considered osteoconductive, osteogenic, and osteoinductive. An allogenic material that provides all three components is the cellular bone matrix allograft (CBM, e.g., ViviGen™, LifeNet Health, Virginia Beach, VA, USA). CBMs have been demonstrated to have viable, lineage committed bone-forming cells within a bony matrix; bone morphogenetic proteins (BMP)-2 and BMP-7; vascular endothelial growth factor (VEGF); and angiogenin. 1 Most studies investigating clinical use of CBMs have only included case reports and limited case series.2-5 To date, there is only one large series demonstrating CBM use in the mandible. 6 There is a paucity of clinical CBM data within the maxillofacial literature.
The purpose of the current study was to determine the success of CBM based maxillary boney reconstruction. The specific aims were to assemble a cohort of subjects who underwent maxillary reconstruction with CBMs, data collection, estimating graft success and measuring associations between the risk factors and graft success. The authors hypothesize that there is a set of one or more variables that are associated with graft success that can be modified to enhance the outcome.
Methods
Study Design and Sample
A retrospective cohort study was designed and implemented using data from subjects who presented to the University of Louisville and were treated with a CBM for maxillary reconstruction from 2019 to 2023. Subjects were excluded if they were not treated with a CBM, data were not complete, or postoperative follow-up time was less than 3 months. This study was approved by the University of Louisville institutional review board (protocol 19.0978). All research activities were conducted as per the World Medical Association Declaration of Helsinki.
Study Variables
The predictor variable was composed of a set of heterogenous variables grouped into demographics, medical history, and etiology. Demographic variables included age (years) and sex (male/female). Medical history variables included history of penicillin (PCN) allergy (yes/no), history of diabetes mellitus (DM) (yes/no) and tobacco use (yes/no). Etiologic variables included bone defects odontogenic cysts (Figure 1), odontogenic tumors, cleft maxilla (Figure 2), ballistic trauma, non-ballistic trauma, osteomyelitis/medication related osteonecrosis of the jaw (MRONJ), or corrective bone surgery (Figure 3). Lastly, adjuncts such as platelet rich fibrin (PRF) and/or the addition of an autograft to the CBM were also recorded.
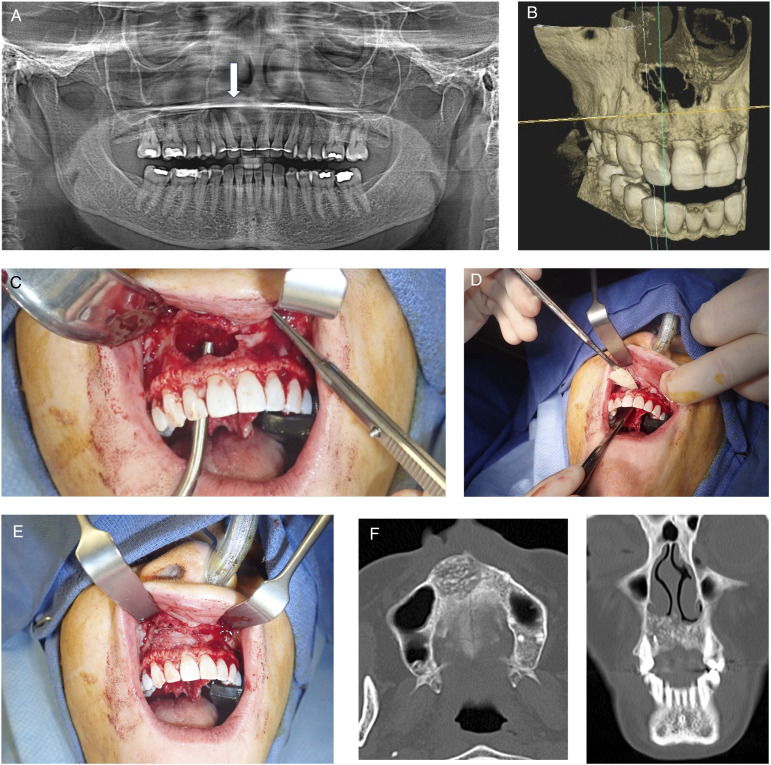
Figure 1.
Subject with anterior maxillary odontogenic cyst. (A) Preoperative panoramic radiograph, arrow points to lesion. (B) 3D reconstruction from cone beam computed tomography scan. (C) Intra operative photo after excision of lesion. (D) Placement of cellular bone matric allograft into defect. (E) Defect filled with cellular bone matrix. (F) CT scan taken 6 months after surgery. Left axial cuts and right coronal cuts. Note defect graft fill and consolidation.
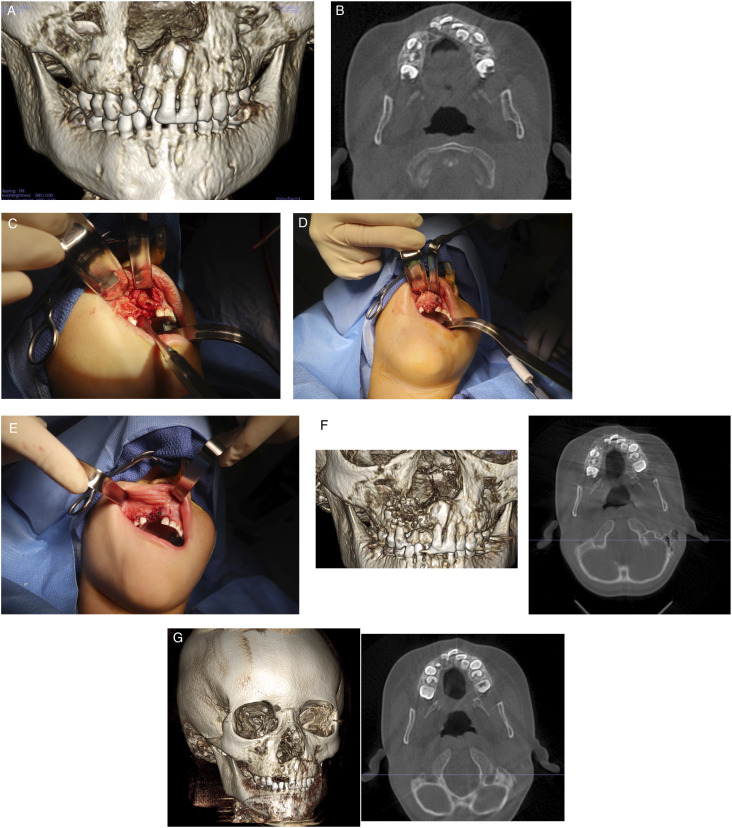
Figure 2.
Bone graft reconstruction of right cleft maxilla with cellular bone matrix and rh-BMP2. (A) Preoperative cone beam computed tomography scan demonstrating defect. (B) Axial scan demonstrating defect. (C) Intraoperative photograph demonstrating cleft maxilla after repair of residual oral nasal fistula. (D) Placement of cellular bone matrix and rh-BMP2 into cleft maxilla. (E) Tension free closure of oral mucosa. (F) Cone beam computed tomography scan one week after surgery, right 3D reconstruction, left axial cut. (G) Cone beam computed tomography scan taken 4 months after surgery, right 3D reconstruction, left axial cuts. Note union of the maxilla.
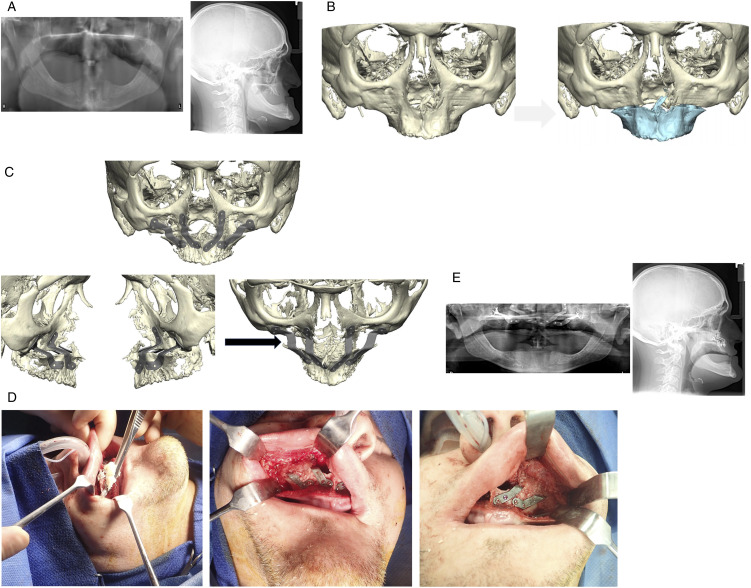
Figure 3.
Subject with edentulous and severely hypoplastic maxilla. (A) Preoperative panoramic radiograph, left, and lateral cephalometric radiograph, right demonstrating severe maxillary hypoplasia. (B) 3D reconstruction demonstrating preoperative position (left) and proposed maxillary advancement, highlighted in blue (right). (C) Rigid internal fixation design. Frontal view (top), lateral view (bottom left), and superior view (bottom right). Note the lack of bony contact after maxillary advancement (arrow). (D) Intra operative view of maxilla after advancement and rigid internal fixation and placement of cellular bone matrix over bony defect. (E) Postoperative panoramic radiograph (left) and lateral cephalometric radiograph (right).
The primary outcome variable was graft success coded yes or no. Success was defined as bony union/defect fill (demonstrated on panoramic radiograph and/or cone beam CT scan) and clinical examination.
Data Collection and Management
Once a research subject was deemed eligible for inclusion in the study their record was reviewed to collect the necessary information for analysis. De-identified data were exported to a standardized database software (Excel™, version 16.54, Microsoft Corporation, Redmond, WA, USA).
Data Analysis
Descriptive statistics were calculated for each variable. Categorical variables are reported using counts and percentages. Continuous variables are reported as the mean and standard deviation. Fisher’s exact tests were used to identify dependence between rates or counts. Univariable logistic regression models were fit to evaluate the association of the continuous predictors (age and follow-up time) with CBM success. All statistical tests were assessed at a significance level of P = 0.05 and were performed using the statistical software R (version 4.3.1, R Foundation for Statistical Computing, Vienna, Austria). Conditional odds ratios and their confidence intervals were obtained using the epitools R package version 0.5-10.1
Results
Descriptive statistics for the cohort are provided in Table 1. A total of 48 subjects were included in the analysis. 23 (48%) subjects were male and 25 (52%) were female. The mean age of all subjects was 43 ± 24 years. Mean post operative follow-up time was 8 ± 7 months. 6 (13%) subjects reported a history of PCN allergy and 4 (8.3%) reported a DM history. No subjects reported a history of alcohol or tobacco use. 29 (60%) subjects had a penicillin type antibiotic administered perioperatively. Most subjects had bone defects reconstructed resulting from benign tumors (10 (21%)), odontogenic cysts (14, (29%)), or corrective bone surgery (10, (21%)). 40 (83%) of subjects were grafted with no concurrent infection. 13 (27%) subjects had defects that were reconstructed with a CBM with the addition of PRF and/or an autograft.
Table 1.
Subject Demographics and Characteristics.
| Characteristic | Value |
|---|---|
| Demographics | |
| n | 48 |
| Sex | |
| Male | 23 (48) |
| Female | 25 (52) |
| Age (years) | 43±24 |
| Postoperative follow-up (months) | 8±7 |
| Medical history | |
| Reported PCN allergy (yes) | 6 (12.5) |
| History of DM (yes) | 4 (8.3) |
| History of tobacco use (no) | 48 (100 |
| History of alcohol use (no) | 48 (100) |
| Antibiotic administered | |
| Cephalosporin | 12 (25) |
| Penicillin type | 29 (60) |
| Clindamycin | 7 (14.5) |
| Etiology | |
| Ballistic trauma | 1 (2.1) |
| Non-ballistic trauma | 7 (15) |
| Benign tumor | 10 (21) |
| Odontogenic cyst | 14 (29) |
| Osteomyelitis/MRONJ | 3 (6.3) |
| Corrective bone surgery defect | 10 (21) |
| Cleft maxilla | 3 (6.3) |
| Infection during graft | |
| Acute | 2 (4.2) |
| Chronic | 6 (13) |
| No infection | 40 (83) |
| Platelet rich fibrin adjunct | 13 (27) |
| Autograft adjunct | 13 (27) |
| Graft outcome | |
| Success | 42 (87.5) |
| Failure | 6 (12.5) |
Note: Data are presented as mean ± SD for continuous data and n (%) for categorical data. Non-ballistic trauma (motor vehicle collision, assault, fall); benign tumor (ameloblastoma, myxoma, ossifying fibroma); odontogenic cyst (odontogenic keratocyst, dentigerous cyst, inflammatory cyst). Abbreviations: PCN, penicillin; DM, diabetes mellitus; MRONJ, medication-related osteonecrosis of the jaw.
The results of the bivariate analysis of risk factors vs graft success are presented in Table 2. Overall, 42 (87.5%) of cases were successful while 6 (12.5%) failed. The association between sex and graft success was nonsignificant (P = 0.67). In addition, age and follow up time were also nonsignificant (P = 0.09 and P = 0.7, respectively). Medical history variables which included a history of PCN allergy (P = 0.573), and DM (P = 1.0) were not associated with graft success. As all subjects reported no alcohol or tobacco use, no association test was conducted for these variables. The perioperative antibiotic used had a significant effect on graft outcome (P = 0.02). Specifically, the use of a penicillin type antibiotic was associated with 12.91 times the odds of graft success when compared to a cephalosporin type. In addition, there was also a significant association between etiology and graft success (P = 0.02). The association of graft infection status and graft outcome was nonsignificant (P = 0.254). Lastly, the use of PRF and/or an autograft had a significant effect on graft outcome (P = 0.039). The use of an autograft and/or PRF had 0.14 time the odds of graft success. Bone taken from during implant placement in a site reconstructed with CBM was completed (Figure 4). The bone was sectioned and stained with Movat’s pentachrome. The photomicrograph demonstrates viable bone with osteocytes, osteoblasts, and osteoclasts.
Table 2.
Bivariate Analysis of Risk Factors vs Graft Success.
| Characteristic | Outcome | OR | 95% CI | P | |
|---|---|---|---|---|---|
| Failure | Success | ||||
| Demographics | |||||
| n | 6 (12.5) | 42 (87.5) | |||
| Sex | 0.51 | 0.04, 3.99 | 0.67 | ||
| Male | 2 (33) | 21 (50) | |||
| Female | 4 (67) | 21 (50) | |||
| Age (years) | 26 ± 17 | 45 ± 24 | 0.96 | 0.9, 1.0 | .09 |
| Postoperative follow-up (months) | 7.7 ± 3.3 | 8.3 ± 7.3 | 0.97 | 0.8, 1.1 | .07 |
| Medical history | |||||
| Reported PCN allergy (yes) | 1 (17) | 5 (12) | .68 | 0.06, 38.27 | 0.57 |
| History of DM (yes) | 0 (0) | 4 (9.5) | inf | 0.08, inf | 1.0 |
| History of tobacco use (no) | 6 (12.5) | 42 (87.5) | |||
| History of alcohol use (no) | 6 (12.5) | 42 (87.5) | |||
| Antibiotic administered | 0.02* | ||||
| Cephalosporin | 4 (67) | 8 (19) | - | - | |
| Penicillin type | 1 (17) | 28 (67) | 12.91 | 1.09, 709.7 | |
| Clindamycin | 1 (17) | 6 (14) | 2.84 | 0.2, 171.76 | |
| Etiology | 0.02* | ||||
| Ballistic trauma | 0 (0) | 1 (2.4) | |||
| Non-ballistic trauma | 1 (17) | 6 (14) | |||
| Benign tumor | 2 (33) | 8 (19) | |||
| Odontogenic cyst | 0 (0) | 14 (33) | |||
| Osteomyelitis/MRONJ | 1 (17) | 2 (4.8) | |||
| Corrective bone surgery defect | 0 (0) | 10 (24) | |||
| Cleft maxilla | 2 (33) | 1 (2.4) | |||
| Infection during graft | 0.254 | ||||
| Acute | 1 (17) | 1 (2.4) | 0.15 | 0.0, 13.44 | |
| Chronic | 0 (0) | 6 (14) | inf | 0.12, inf | |
| No Infection | 5 (83) | 35 (83) | - | - | |
| Platelet rich fibrin adjunct (yes) | 4 (67) | 9 (21) | 0.14 | 0.01, 1.18 | 0.039* |
| Autograft adjunct (yes) | 4 (67) | 9 (21) | 0.14 | 0.01, 1.18 | 0.039* |
Note: Data are presented as mean ± SD for continuous data and n (%) for categorical data. Non-ballistic trauma (motor vehicle collision, assault, fall); benign tumor (ameloblastoma, myxoma, ossifying fibroma); odontogenic cyst (odontogenic keratocyst, dentigerous cyst, inflammatory cyst). Abbreviations: PCN, penicillin; DM, diabetes mellitus; MRONJ, medication-related osteonecrosis of the jaw.
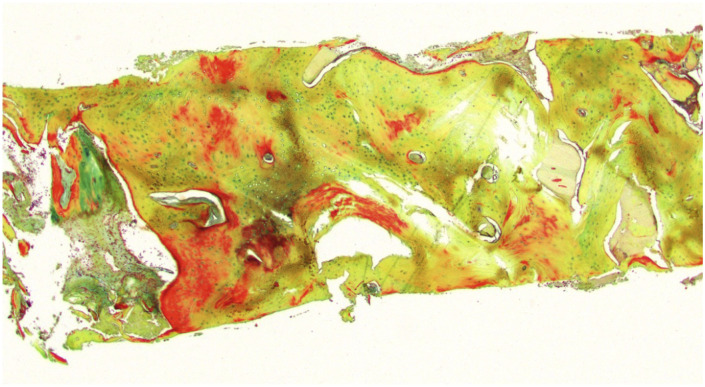
Figure 4.
Photomicrograph of bone core taken during endosseous dental implant in defect reconstructed with cellular bone matrix. Movat’s pentachrome staining of bone (nuclei, black to dark-bluish gray; osteoid, red or yellow; mineralized bone, yellow).
Discussion
Reconstruction of the maxilla is complex, even for seasoned maxillofacial surgeons. The gold standard of maxillary bone reconstruction is vascularized and non-vascularized autologous bone. The use of tissue engineering applications and new advanced allografts, such as CBMs, are challenging this notion.
CBMs have been used extensively in spine and orthopedic surgery.7-10 However, data within the maxillofacial complex is lacking. This series on maxillary reconstruction, along with the primary authors investigation on CBM based mandibular reconstruction 6 are the largest series on the subject. Overall, 42 (88%) of 48 cases were successful, which was defined as bony union/defect fill (demonstrated on panoramic radiograph and/or cone beam CT scan) and clinical examination. Like our series on CBM based mandibular reconstruction, 6 this cohort represented varied and complex clinical scenarios. Etiologies included ballistic injuries, non-ballistic injuries, defects encountered during corrective bone surgery, cleft maxilla, and pathology such as benign cysts, tumors, and osteomyelitis/MRONJ. Interestingly, the etiology (P = 0.02), perioperative antibiotic administered (P = 0.02), and graft adjuncts (PRF and/or autograft), P = 0.039)) influenced CBM graft success.
In general, companies that produce CBMs do so via proprietary tissue processing techniques. The manufacturing process removes all material that could illicit an immune response generated by bone marrow components. There are several CBMs currently on the market. Govoni et al 11 provides a nice review of commercially available CBMs. In general, CBMs can differ in cell type preserved, cell amount, cellular viability after thawing, tissue processing/formulation, and cryoprotectant. 11
The cell types that are available in the different CBMs are wide ranging and include mesenchymal stem cells (MSCs), osteoprogenitor cells (OPCs), or lineage committed bone cells such as osteoblasts (OBs). MSCs are non-hemopoietic cells found in the bone marrow stroma and have the ability to differentiate into a variety of tissues based on the microenvironmental cues the cell encounters.12,13 Furthermore, OPCs are located on the endosteal and periosteal surface of the bone and within the Haversian canal system. 11 In general, OPCs demonstrate differentiative potential similar to that of MSCs. 14 Conversely, another approach is to use methods that washout bone marrow contents and focus on cells are more differentiated such as OBs. OBs are differentiated cuboidal cells that are responsible for bone formation, when bone formation is completed they are trapped within the matrix and are terminally differentiated into osteocytes. 11 Interestingly, cryopreservation has been demonstrated to reduce the immunogenic potential of allogenic lineage-committed bone forming cells.11,15 Post thaw cellular amount and viability is also an important consideration. In general, products that contain MSCs and OPCs (e.g., Trinity Elite™ and Trinity Evolution™, Orthofix Medical Inc., Lewisville, TX, USA) demonstrate a larger absolute cell count (>750,000/cc) and a post thaw viability of approximately 70%.11,16-18 The CBM investigated in the current study (ViviGen™, LifeNet Health, Virginia Beach, VA, USA) is the only CBM focused on lineage committed bone forming cells, specifically, OBs. Considering this, the overall cell count per cc is less (approximately >16,000/cc) with a post thaw viability exceeding 96%.19,20 Even though the absolute cell count is less, OBs are committed to forming bone, as compared to the MSCs which could have more varied differentiation paths.
When comparing the current investigation to the primary authors previous study on CBM based mandibular reconstruction several notable differences are appreciated. First, the overall success is higher in maxillary reconstruction vs mandibular reconstruction 6 (88% vs 73.7%). Reconstruction of bone defects of the maxilla and mandible are fundamentally different. Importantly, most defects encountered in the current study were bone cavities reconstructed after extirpation of odontogenic cysts/tumors, representing Brown class I defects. Which in general may have a high likelihood of success regardless of reconstruction modality. Another difference between the investigations was PCN allergy and subsequent treatment with clindamycin influenced case success in CBM based mandibular reconstruction. 6 The data presented here revealed that reported history of PCN did not correlate with graft success; however, treatment with cephalosporin did. The use of a penicillin type antibiotic was associated with 12.91 times the odds of graft success when compared to a cephalosporin type. PCN type antibiotics have the best coverage for oral and sinonasal flora. The other antibiotics selected likely didn’t cover the microbial flora of this area leading to infections that required wash out or lead to wound break down and graft failure. It is important to note that these observations should not be over interpreted as the sample size is small.
Data and literature on CBM based maxillary reconstruction is essentially non-existent. However, the use of allogenic block and particulate bone grafts (eg Puros™, Zimmer Biomet, Warsaw, IN, USA) has been well studied with and without adjuncts such as rh-BMP2. More specifically, allogenic bone graft material has demonstrated predictable results in dentoalveolar reconstruction in preparation for dental implants. 21 Furthermore, particulate allogenic bone grafts have been used in cleft maxilla reconstruction for many years. 22 However, no direct comparison exists between traditional allogenic particulate materials vs CBMs. Thus, it is not clear how much benefit the additional of viable cells play. Regardless, with an overall success rate of 88% in a variety of clinical scenarios, CBMs do yield predictable results in maxillary bone reconstruction. Interestingly, the presence of graft adjuncts such as PRF or an autograft were correlated with higher likelihood of graft failure. This is likely confounded because adjuncts were more likely to be used in more complex reconstructive scenarios, which would have likely had a higher risk of failure in general.
The strengths of the current study are the relatively large cohort relative to the available literature, indeed, this is the largest series of CBM based reconstruction in craniomaxillofacial surgery to date. The major limitation of this study is the fact that it is retrospective.
Conclusion
Here, we demonstrate that CBM based bone reconstruction of the maxilla is predicable in a variety of clinical scenarios. Furthermore, clinicians should pay close attention to what perioperative is prescribed and use PCN based medications when possible. Additional work is indicated for comparisons to autogenous bone and other CBM products.
Footnotes
Dr. Kushner and Dr. Marschall serve as consultants for LifeNet Health.
Funding: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Jeffrey S. Marschall https://orcid.org/0009-0002-7123-232X
References
- 1.Gianulis E, Wetzell B, Scheunemann D, et al. Characterization of an advanced viable bone allograft with preserved native bone-forming cells. Cell Tissue Bank. 2023;24(2):417-434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marschall JS, Kushner GM, Flint RL, Jones LC, Alpert B. Immediate reconstruction of segmental mandibular defects with nonvascular bone grafts: a 30-year perspective. J Oral Maxillofac Surg. 2020;78(11):2099.e1. [DOI] [PubMed] [Google Scholar]
- 3.Pontell ME, Barahimi B, Golinko MS. Managing bilateral oro-sino-orbital fistulae in the setting of bilateral tessier IV clefts. J Craniofac Surg. 2022;33(1):e73-e76. [DOI] [PubMed] [Google Scholar]
- 4.Ryu B, Abraham C, Polido WD. Treatment of mandibular non-union using patient specific crib cage plates and cellular bone allograft: a case report. Craniomaxillofac Trauma Reconstr Open. 2021;6:247275122110059. [Google Scholar]
- 5.Alfi DM, Hassan A, East SM, Gianulis EC. Immediate mandibular reconstruction using a cellular bone allograft following tumor resection in a pediatric patient. FACE. 2021;2(4):490-495. [Google Scholar]
- 6.Marschall JS, Davis SS, Jones L, Kushner GM. Are cellular bone matrix allografts a viable option for mandibular tissue engineering and reconstruction? J Oral Maxillofac Surg. 2024;82(9):1163-1175. [DOI] [PubMed] [Google Scholar]
- 7.Elgafy H, Wetzell B, Gillette M, et al. Lumbar spine fusion outcomes using a cellular bone allograft with lineage-committed bone-forming cells in 96 patients. BMC Muscoskel Disord. 2021;22(1):699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moran TE, Sequeira S, Cooper MT, Park J. A retrospective analysis of outcomes from foot and ankle arthrodesis and open reduction and internal fixation using cellular bone allograft augmentation. Foot Ankle Spec. 2022;15(4):312-320. [DOI] [PubMed] [Google Scholar]
- 9.Jones CP, Loveland J, Atkinson BL, Ryaby JT, Linovitz RJ, Nunley JA. Prospective, multicenter evaluation of allogeneic bone matrix containing viable osteogenic cells in foot and/or ankle arthrodesis. Foot Ankle Int. 2015;36(10):1129-1137. [DOI] [PubMed] [Google Scholar]
- 10.Pinter ZW, Elder BD, Kaye ID, et al. A review of commercially available cellular-based allografts. Clin Spine Surg. 2022;35(1):E77-E86. [DOI] [PubMed] [Google Scholar]
- 11.Govoni M, Vivarelli L, Mazzotta A, Stagni C, Maso A, Dallari D. Commercial bone grafts claimed as an alternative to autografts: current trends for clinical applications in orthopaedics. Materials. 2021;14(12):3290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lutolf MP, Hubbell JA. Synthetic biomaterials as instructive extracellular microenvironments for morphogenesis in tissue engineering. Nat Biotechnol. 2005;23(1):47-55. [DOI] [PubMed] [Google Scholar]
- 13.Mendez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466(7308):829-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Corradetti B, Taraballi F, Powell S, et al. Osteoprogenitor cells from bone marrow and cortical bone: understanding how the environment affects their fate. Stem Cell Dev. 2015;24(9):1112-1123. [DOI] [PubMed] [Google Scholar]
- 15.Yu HB, Shen GF, Wei FC. Effect of cryopreservation on the immunogenicity of osteoblasts. Transplant Proc. 2007;39(10):3030-3031. [DOI] [PubMed] [Google Scholar]
- 16.Peppers TA, Bullard DE, Vanichkachorn JS, et al. Prospective clinical and radiographic evaluation of an allogeneic bone matrix containing stem cells (Trinity Evolution® Viable Cellular Bone Matrix) in patients undergoing two-level anterior cervical discectomy and fusion. J Orthop Surg Res. 2017;12(1):67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vanichkachorn J, Peppers T, Bullard D, Stanley SK, Linovitz RJ, Ryaby JT. A prospective clinical and radiographic 12-month outcome study of patients undergoing single-level anterior cervical discectomy and fusion for symptomatic cervical degenerative disc disease utilizing a novel viable allogeneic, cancellous, bone matrix (trinity evolution) with a comparison to historical controls. Eur Spine J. 2016;25(7):2233-2238. [DOI] [PubMed] [Google Scholar]
- 18.Musante DB, Firtha ME, Atkinson BL, Hahn R, Ryaby JT, Linovitz RJ. Clinical evaluation of an allogeneic bone matrix containing viable osteogenic cells in patients undergoing one- and two-level posterolateral lumbar arthrodesis with decompressive laminectomy. J Orthop Surg Res. 2016;11(1):63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Divi SN, Mikhael MM. Use of allogenic mesenchymal cellular bone matrix in anterior and posterior cervical spinal fusion: a case series of 21 patients. Asian Spine J. 2017;11(3):454-462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hall JF, McLean JB, Jones SM, Moore MA, Nicholson MD, Dorsch KA. Multilevel instrumented posterolateral lumbar spine fusion with an allogeneic cellular bone graft. J Orthop Surg Res. 2019;14(1):372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Le BT, Borzabadi-Farahani A. Simultaneous implant placement and bone grafting with particulate mineralized allograft in sites with buccal wall defects, a three-year follow-up and review of literature. J Cranio-Maxillo-Fac Surg. 2014;42(5):552-559. [DOI] [PubMed] [Google Scholar]
- 22.Nique T, Fonseca RJ, Upton LG, Scott R. Particulate allogeneic bone grafts into maxillary alveolar clefts in humans: a preliminary report. J Oral Maxillofac Surg. 1987;45(5):386-392. [DOI] [PubMed] [Google Scholar]