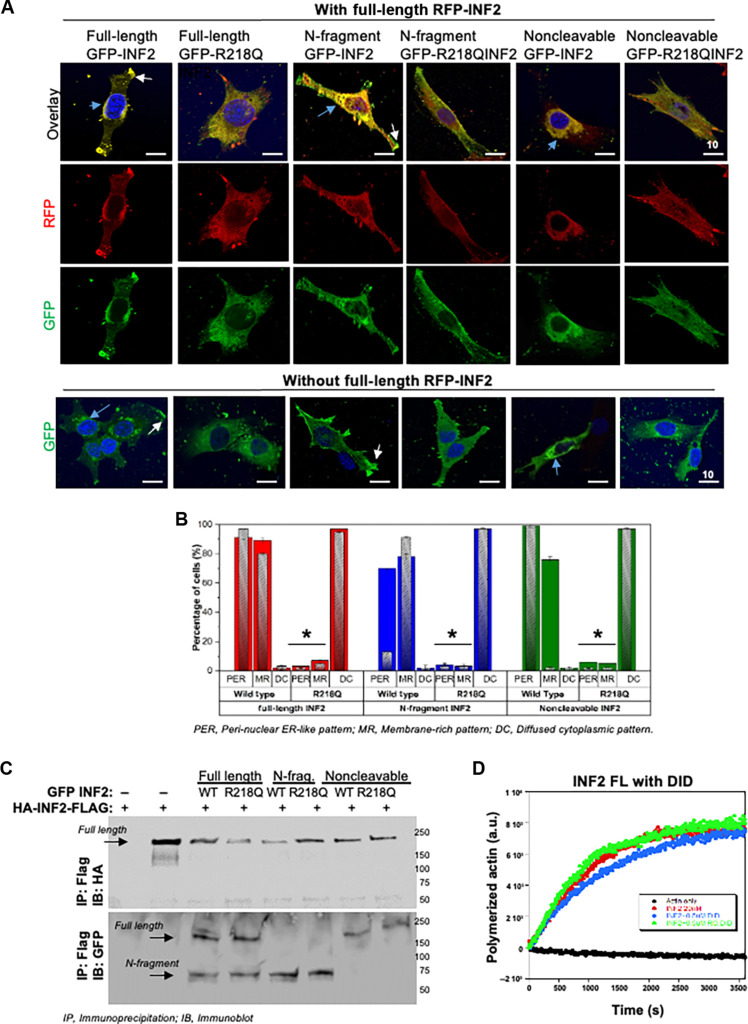
Fig. 3. R218Q pathogenic mutation leads to altered localization and activity of wild-type INF2.
[(A) and (B)] Localization analysis of wild-type INF2 in the presence of R218Q INF2. Wild-type and R218Q GFP-tagged INF2 forms were coexpressed with or without RFP-tagged wild-type full-length INF2. Wild-type full-length RFP-INF2 showed a peri-nuclear ER-like pattern (blue arrow) with some membrane regions (white arrow, membrane region localization). The coexistence of R218Q GFP INF2 forms (full-length, N-fragment, and noncleavable) alters this localization to a diffused cytoplasmic pattern. (A) Quantification of wild-type full-length RFP INF2 localization pattern in podocytes. Color bars correspond to the coexpression of GFP- and RFP-tagged INF2 conditions. Red bars, coexpression with full-length GFP-INF2; blue bars, coexpression with N-fragment GFP-INF2; green bars, coexpression with a noncleavable GFP-INF2. Inlet gray bars represent their respective controls (without RFP-tagged INF2 expression). The peri-nuclear ER-like and membrane-rich localization pattern of wild-type RFP-INF2 is altered to a diffused cytosolic pattern of localization by the R218Q GFP-INF2 presence (*P < 0.01; Student’s t test). (B) Representative cell images for wild-type full-length RFP-INF2 localization with various GFP-INF2 forms. Scale bar, 10 μm. (C) Interaction analysis of wild-type INF2 with R218Q INF2. Coimmunoprecipitation of wild-type HA-INF-FLAG with different GFP-tagged INF2 expression forms. GFP-tagged and HA-tagged INF2 forms were cotransfected in 293T cells. Cell lysates were pulled down using the FLAG tag and blotted for HA. HA immunoblot confirmed the immunoprecipitation of wild-type full-length INF2. GFP immunoblot showed an interaction of wild-type full-length INF2 with both wild-type and R218Q mutant forms of N-fragment, full-length, and noncleavable INF2. Each immunoblot is representative of three independent experiments with similar results. (D) Pyrene actin polymerization assays. Actin polymerizing activity of 20 nM full-length wild-type INF2 was assessed with 0.5 μM wild-type and R218Q DID regions, respectively. Neither the wild-type nor the R218Q-DID region had any effect in the actin polymerizing activity of full-length INF2.