Fig. 4. Analysis of TCR-engineered CD4+ T cells in vitro did not reliably predict therapeutic value in vivo.
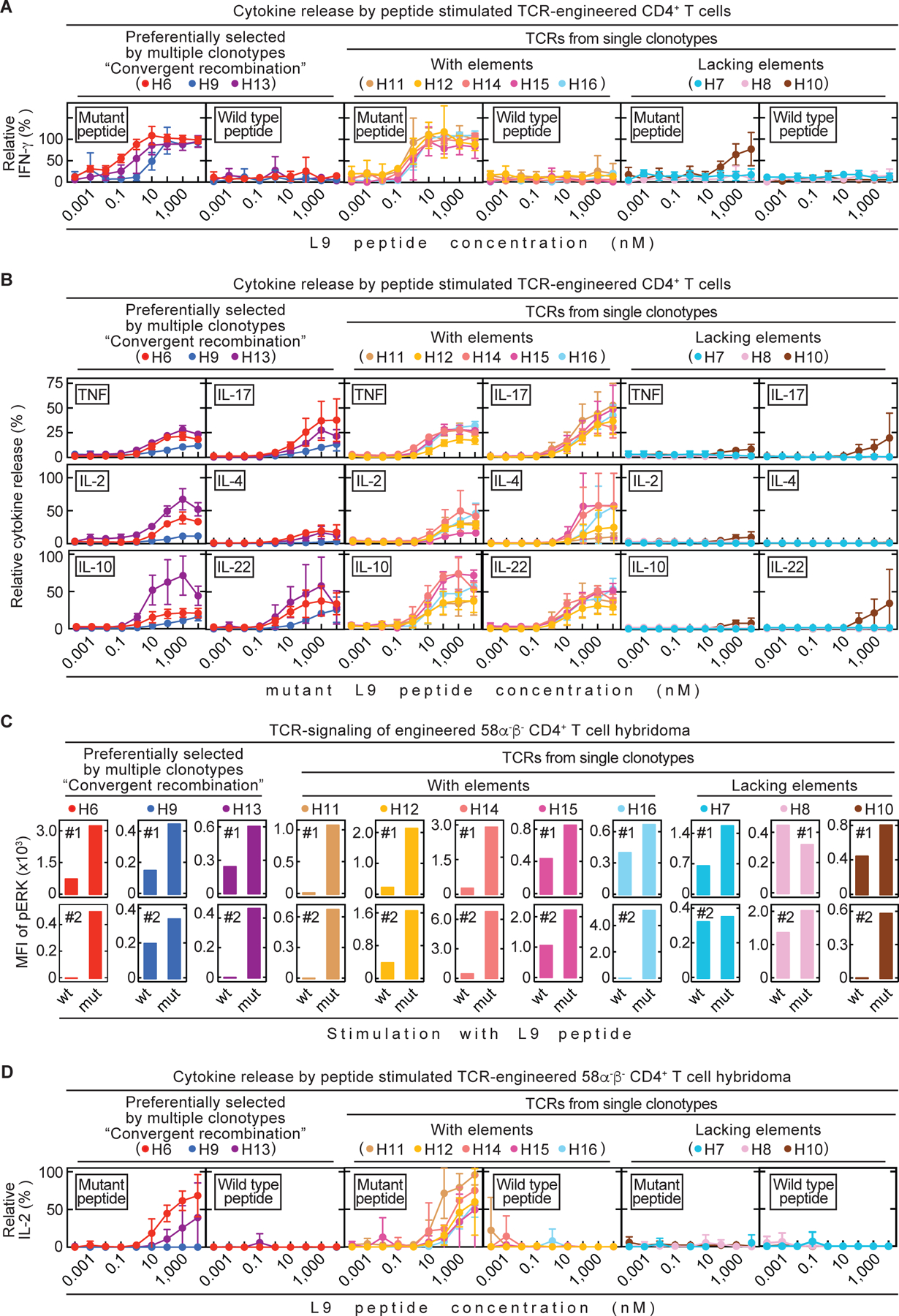
All 11 TCRs were tested in vitro. (A – B) Spleens from C3H CD8-/- mice were used as source for CD4+ T cells. TCR-engineered CD4+ T cells were co-cultured 24 h with C3H/HeN spleen cells and various mutant or wild type L9 peptide concentrations. Data are means ± standard deviation and compiled from two independent experiments. (A) Supernatants were analyzed for IFN-γ concentrations by ELISA. (B) Supernatants were analyzed for various cytokines by flow cytometry. (C – D) TCR-engineered 58α-β- CD4+ T cell hybridomas were used for co-cultures together with LK35 B cell hybridoma as antigen presenting cell (APC) of either mutant or wild type L9 peptide. (C) Phosphorylation of ERK1/2, as a measure of TCR-signaling, was determined by flow cytometry (Mean fluorescent intensity (MFI)). Life, TCR β-chain positive 58α-β- cells were analyzed. Shown are both (#1, #2) independently performed experiments. (D) Co-cultures were performed for 24 h using various mutant or wild type L9 peptide concentrations. Supernatants were analyzed for IL-2 by ELISA. Data are means ± standard deviation and compiled from two independent experiments.
