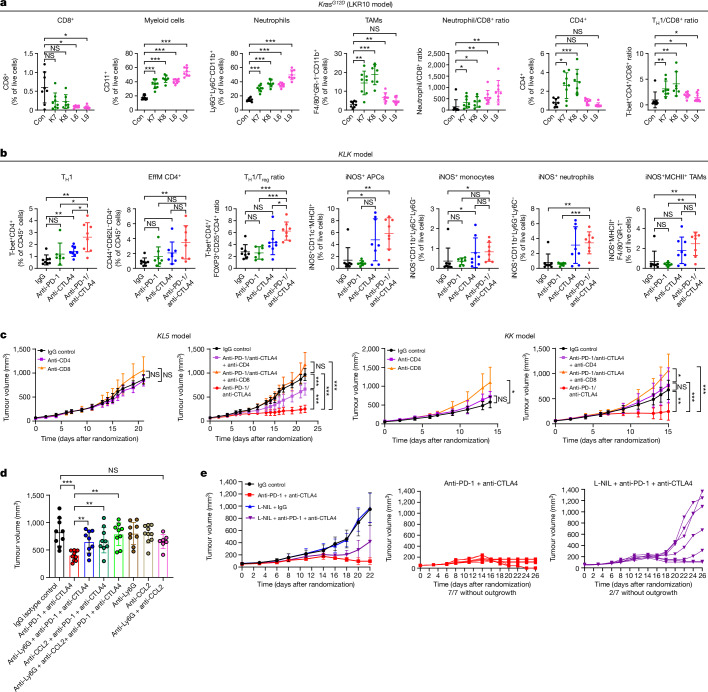
Fig. 4. Innate immune cells and CD4+ effectors are crucial mediators of dual anti-PD-1/anti-CTLA4 efficacy in Stk11- and/or Keap1-deficient models of KRAS-mutant NSCLC.
a, FACS-based enumeration of immune cell subsets in Keap1-deficient (K7, K8), Stk11-deficient (L6, L9) or isogenic Keap1 and Stk11-proficient LKR10 (control, Con) allograft tumours reveals a myeloid-cell-enriched and CD8+ T-cell-depleted suppressive TIME with relative sparing of TH1 CD4+ cells. Data are mean ± s.d. (n = 7–8 mice per group). TAMs, tumour-associated macrophages. b, FACS-based assessment of single and dual ICB-induced changes in the abundance of distinct T cell (left panels) and myeloid cell (right panels) subsets in the microenvironment of the Stk11- and Keap1-deficient KLK model. Data are mean ± s.d. (n = 7–8 mice per group). EffM, effector memory cells. c, Effect of CD4+ or CD8+ depletion on the in vivo growth kinetics of KL5 and KK models in the absence of treatment or with dual anti-PD-1/anti-CTLA4 therapy (n = 7–8 mice per group). Comparison of tumour volume was performed at the time point at which the first mouse in any treatment group reached a tumour volume ≥ 1,500 mm3. d, The anti-tumour activity of dual PD-1/CTLA4 ICB in the KL5 model is dependent on innate immune cells (n = 7–10 mice per group). Tumour volume is shown for the indicated treatment arms. e, iNOS inhibition curtails the anti-tumour efficacy of dual ICB in the KLK model (n = 7–8 mice per group). Comparison of tumour volume was performed at the time point at which the first mouse in any treatment group reached a tumour volume ≥ 1,200 mm3. Individual tumour growth trajectories in the anti-PD-1 + anti-CTLA4 (red) and L-NIL + anti-PD-1 + anti-CTLA4 (purple) treatment arms are also shown. Mann–Whitney U test was used for all pairwise statistical comparisons. Data are mean ± s.d. Statistical significance is indicated (*P ≤ 0.05, **P ≤ 0.01, ***P ≤ 0.001).