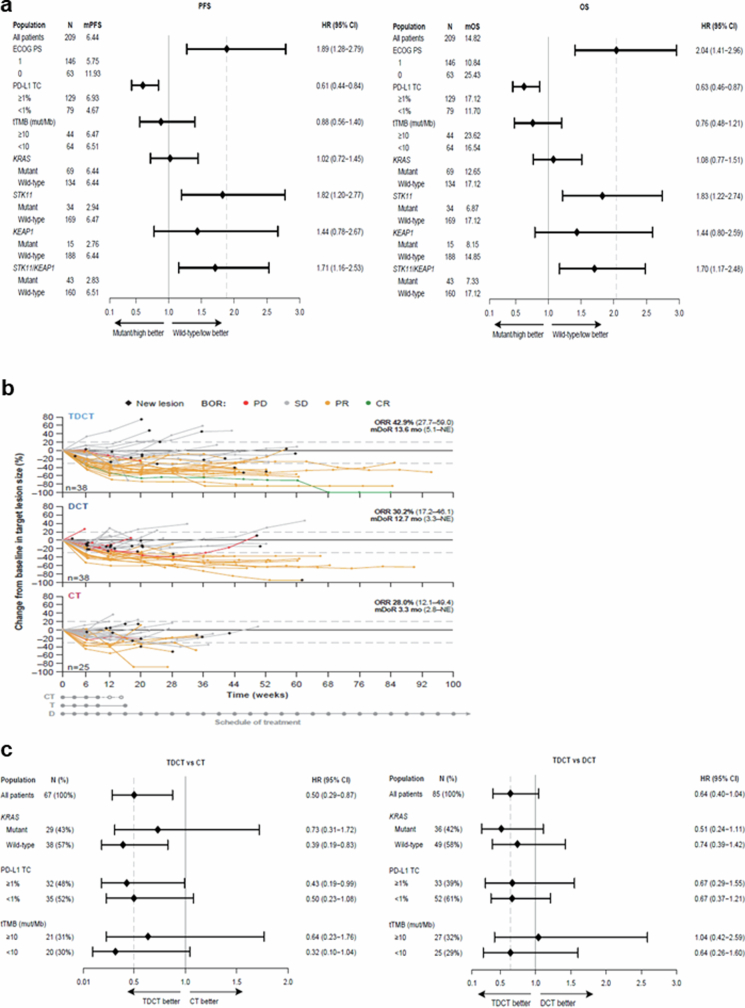
Extended Data Fig. 2. Clinical outcomes in patient subgroups in the POSEIDON clinical trial.
a. PFS (left) and OS (right) with DCT in patient subgroups defined by clinical and molecular characteristics. HRs and 95% CIs were estimated using unstratified Cox proportional hazards models. The analysis of PFS was based on a data cut-off date of July 24, 2019 and the analysis of OS was based on a data cut-off date of March 12, 2021. b. Spider plots, depicting patient-level % change compared to baseline in the size of target lesion(s) (per RECIST v1.1) in patients with STK11 and/or KEAP1-mutated nsNSCLC treated with TDCT (top), DCT (middle) and CT (bottom). Individual trajectories are colour-coded based on best overall response. Only patients with both a baseline and at least one available post-baseline target lesion measurement are included. ORR and mDoR are based on confirmed objective responses by BICR. The analysis was based on a data cut-off date of July 24, 2019. c. OS in molecularly defined subgroups of patients with STK11MUT and/or KEAP1MUT metastatic nsNSCLC treated with TDCT vs CT (left) and TDCT vs DCT (right). HRs and 95% CIs were estimated using unstratified Cox proportional hazards models. The analysis was based on a data cut-off date of March 12, 2021.