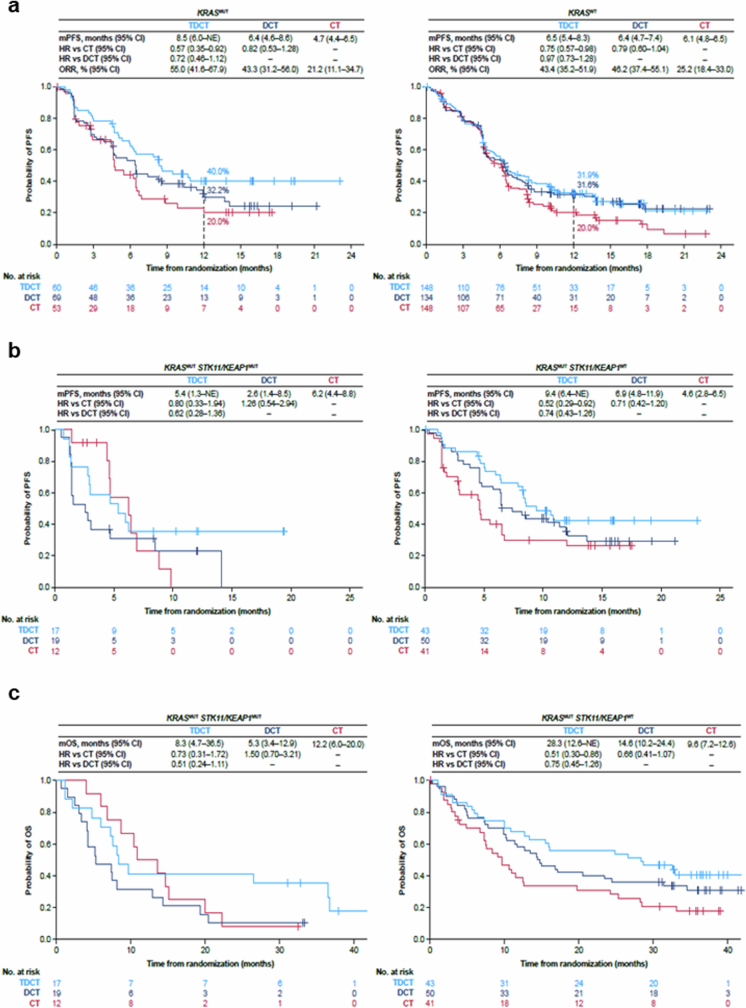
Extended Data Fig. 4. Clinical outcomes in patients with KRAS-mutated nsNSCLC in the phase III POSEIDON clinical trial.
a. Kaplan–Meier estimates of PFS according to BICR per RECIST v1.1 with TDCT (light blue curve) vs DCT (dark blue curve) vs CT (red curve) in patients bearing KRASMUT (left panel) and KRASWT (right panel) metastatic nsNSCLC. Landmark 12-month PFS rates in each of the treatment arms are also shown (dotted lines). b,c. Kaplan–Meier estimates of PFS according to BICR per RECIST v1.1 (b) and OS (c) with TDCT vs DCT vs CT in patients bearing KRASMUT; STK11MUT and/or KEAP1MUT (left panel) and KRASMUT; STK11WT and KEAP1WT (right panel) metastatic nsNSCLC. PFS and ORR analyses were based on a data cut-off date of July 24, 2019. OS analyses were based on a data cut-off date of March 12, 2021. HRs and 95% CIs were estimated using unstratified Cox proportional hazards models.