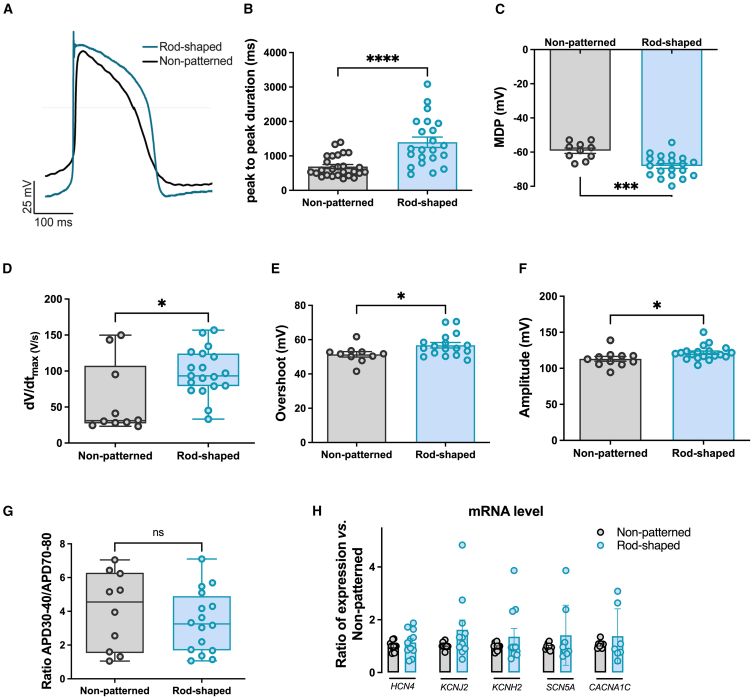
Figure 3.
Improved electrophysiological properties of rod-shaped micropatterned hiPSC-CMs
(A) Representative overlayed ventricular-like AP traces recorded from non-patterned and rod-shaped micropatterned hiPSC-CMs 10 days after seeding.
(B and C) Bar graphs quantifying (B) peak-to-peak interval duration averaged from spontaneously beating hiPSC-CMs and (C) membrane diastolic potential measured in non-patterned and rod-shaped CMs, paced at 1 Hz.
(D) Box whisker blot showing maximum upstroke velocity dV/dtmax calculated in non-patterned and rod-shaped hiPSC-CMs, paced at 1 Hz.
(E and F) Bar graphs quantifying (E) upstroke overshoot analyzed from APs recorded in non-patterned and rod-shaped hiPSC-CMs which were paced at 1 Hz and (F) depolarization amplitude averaged from APs paced at 1 Hz and recorded in non-patterned and rod-shaped hiPSC-CMs.
(G) APD30-40/APD70-80 ratio, which reflects the plateau phase ratio in non-patterned and rod-shaped hiPSC-CMs paced at 1 Hz.
(H) RNA expression of HCN4, KCNJ2, KCNH2, SCN5A, and CACNA1C measured by SYBR green in non-patterned and rod-shaped hiPSC-CMs. CTs were normalized to RPL32, and the ratio of rod-shaped to non-patterned was calculated for each cardiac differentiation. CTs, threshold cycle.
Data are expressed as mean ± SEM. For (B–G), n = 10–26 from 4 independent experiments. For (H), n = 3 independent experiments from 7 differentiations per condition. Mann-Whitney: ns, p > 0.05, ∗p < 0.05, ∗∗∗p < 0.001, and ∗∗∗∗p < 0.0001 vs. non-patterned hiPSC-CMs.