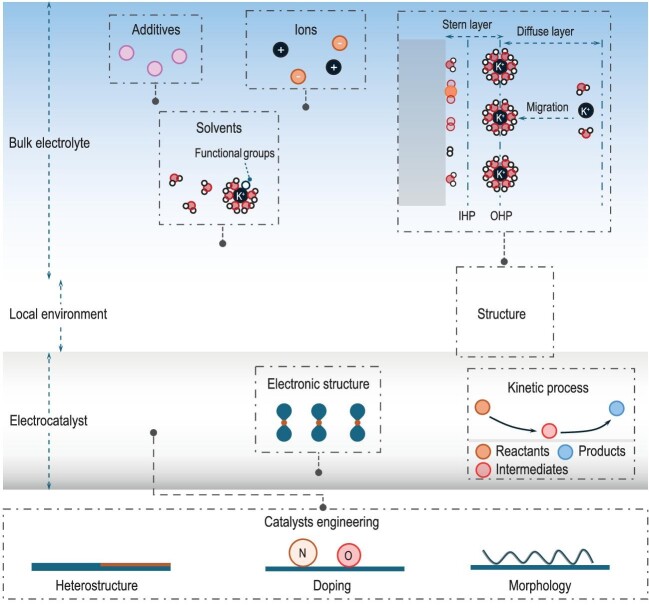
Figure 1.
The electrochemical system can be divided into three parts: electrocatalysts, local environment, and bulk electrolyte. The electronic structure of electrocatalysts affect the kinetics process at the electrolyte-electrode interface, while the electrolyte system influences the diffusion rate of species. Meanwhile, the changes in electrocatalysts and electrolyte will result in the dynamic evolution of the local environment. The structure of the local environment is composed of a Stern layer and Diffuse layer. The Stern layer can be subdivided into IHP (inner Helmholtz plane) and OHP (outer Helmholtz plane). The reactants will interface with electrodes and evolve in this region.