Abstract
The concept of this review underscores a significant shift towards sustainable agricultural practices, particularly from the view point of microbial biotechnology and nanotechnology. The global food insecurity that causes increasing ecological imbalances is exacerbating food insecurity, and this has necessitated eco-friendly agricultural innovations. The chemical fertilizers usage aims at boosting crop yields, but with negative environmental impact, thus pushing for alternatives. Microbial biotechnology and nanotechnology fields are gaining traction for their potential in sustainable agriculture. Endophytic fungi promise to synthesize nanoparticles (NPs) that can enhance crop productivity and contribute to ecosystem stability. Leveraging on endophytic fungi could be key to achieving food security goals. Endophytic fungi explore diverse mechanisms in enhancing plant growth and resilience to environmental stresses. The application of endophytic fungi in agricultural settings is profound with notable successes. Hence, adopting interdisciplinary research approaches by combining mycology, nanotechnology, agronomy, and environmental science can meaningfully serve as potential pathways and hurdles for the commercialization of these biotechnologies. Therefore, setting regulatory frameworks for endophytic nanomaterials use in agriculture, by considering their safety and environmental impact assessments will potentially provide future research directions in addressing the current constraints and unlock the potential of endophytic fungi in agriculture.
Keywords: Antifungal, Biogenesis, Nano-biotechnology, Nano-enabled agriculture, Nano-structure, Plant yield component
Highlights
-
•
Culturable endophytic fungi with the ability to synthesize nanoparticles.
-
•
Nanotechnology in the reality of microbial world exploration.
-
•
Importance of plant microbes in agricultural biotechnology.
-
•
The potential of endophytic microbes in agriculture, pharmaceutical, and industry.
1. Introduction
Nanotechnology represents a pivotal advancement in the contemporary science of manipulating matter at the atomic and molecular scale, and this has revolutionized numerous fields with its applications spanning agriculture, medicine, engineering, chemistry, mathematics, physics, and environmental science [1]. This technology enables the creation of NPs with sizes ranging from 1 to 100 nm, offering unique properties and functionalities that are leveraged in various applications [2]. The widespread applicability of nanotechnology highlights its transformative impact across multiple disciplines, emphasizing the need for sustainable and environmentally friendly approaches to nanoparticle synthesis [3]. Traditionally, nanoparticle synthesis has relied on physical and chemical methods, which often involve hazardous chemicals and high energy consumption. These conventional techniques, while effective in producing large quantities of uniform NPs, pose significant health and environmental risks [4]. In response, the field has seen a shift towards green chemistry approaches, which emphasize the use of biological systems for nanoparticle synthesis and these have emerged as preferable alternatives to traditional physical and chemical processes [5]. The biological methods are increasingly favored for their cost-effectiveness, environmental friendliness, and reduced reliance on toxic substances [6]. This shift aligns with broader efforts to minimize ecological impact and enhance sustainability in nanotechnology. In contrast, chemical methods, while often yielding large quantities of uniform NPs, involve toxic substances and energy-intensive processes, raising significant ecological concerns [7].
Myco-nanotechnology represents a promising subset of nanotechnology that focuses on using fungi for nanoparticle synthesis [8]. This approach leverages the metabolic capabilities of fungi to produce NPs in a more eco-friendly, sustainable, and energy-efficient manner compared to traditional chemical processes [9]. This approach aims to minimize energy consumption and environmental impact, addressing the challenges posed by conventional methods. Specifically, the use of NPs derived from biological systems holds the potential for enhancing agricultural productivity and ensuring food security sustainably [10].
Nanoparticles, characterized by their size range of 1–100 nm, possess unique properties that are advantageous for various applications [11]. Among the nanoparticle production methods—physical, chemical, and biological—the latter, particularly involving endophytic fungi, shows significant promise for crop improvement and plant health [12]. Recent research highlights the catalytic efficiency, chemical stability, and conductivity of NPs synthesized from plant-associated endophytic fungi [13,14].
Studies by mycologists underscore the potential of endophytic fungi as reliable sources of nanomaterials with biological significance [15,16]. These fungi are noted for their tolerance to heavy metals and their ability to produce silver NPs [17]. Fungi are favored over other microorganisms for nanoparticle production due to the manageable nature of their biomass. NPs are typically recognized for their high surface area and biological relevance, including anticancer, antidiabetic, antioxidant, and antibacterial activities [14,18].
Nanoparticle synthesis can be broadly categorized into top-down and bottom-up approaches. The top-down approach involves mechanical grinding/physical breaking down of bulk materials into nanoscale particles and stabilizing the materials using colloidal agents [19]. In contrast, the biological synthesis of NPs—falling under the bottom-up approach—involves building NPs from atomic or molecular precursors, often utilizing biological systems, such as plant extracts and microorganisms like endophytic fungi [20]. Among these, the biological synthesis of NPs is categorized into intracellular and extracellular methods. The extracellular method is often preferred due to its simplicity and ease of purification [21]. Intracellular synthesis involves the uptake of metal ions into fungal cells, where enzymes facilitate nanoparticle formation, typically resulting in smaller particles compared to extracellular synthesis [22].
Fungi, particularly endophytic fungi, are of significant interest due to their ability to produce NPs with unique properties and applications [23]. The use of endophytic fungi in nanoparticle synthesis is advantageous due to their high tolerance to heavy metals and their potential for producing NPs such as silver with notable biological activities [17]. Endophytic fungi, which inhabit plant tissues without causing disease, offer promising avenues for producing biocontrol agents and bioactive nanomaterials [24]. These fungi can interact with plants in mutualistic, parasitic, or symbiotic relationships, contributing to plant health and productivity [25]. They can produce NPs through intracellular or extracellular processes, with extracellular synthesis typically resulting in larger and more easily recoverable NPs. The ability of endophytic fungi to synthesize NPs with diverse functions, such as antimicrobial and antioxidant properties, makes them valuable for applications in agriculture, food security, and medicine [26]. The role of endophytic fungi extends to enhancing crop production through their ability to convert metal ions into useful elements [27]. The biological functions of NPs produced by these fungi—including silver, cobalt, iron, copper, nickel, and zinc—have been documented [28,29]. As researchers continue to explore the synthesis of NPs from plant-associated microbes, the focus on biological methods will be crucial for maximizing the benefits of these nanomaterials in agriculture and environmental sustainability. Research into endophytic fungi underscores their potential and prospects as bio-nano factories capable of producing environmentally friendly NPs with significant agricultural benefits under climatic conditions [30].
The integration of nanotechnology with green chemistry principles, particularly through the use of biological systems, such as endophytic fungi, represents a major advancement in the sustainable production of NPs [29]. This approach not only addresses the environmental and health concerns associated with traditional methods but also opens new possibilities for enhancing crop productivity, food security, and medical applications [31]. Future research should focus on optimizing the synthesis processes, exploring the full range of NPs produced by fungi, and expanding their applications to maximize the benefits of this innovative technology. Hence, this review provides a comprehensive overview of endophytic fungi and their role in nanoparticle synthesis, highlighting their potential contributions to plant and soil health and offering insights into future research directions.
2. Plant microbiome and application in agricultural biotechnology
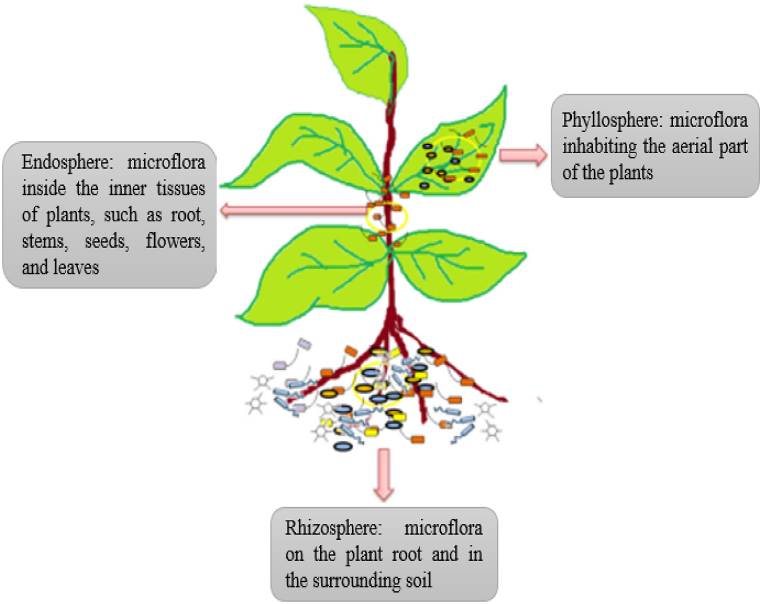
Previous studies have revealed the complex assemblage of endophytic fungi in diverse plant compartments, playing vital roles in improving overall plant performance and adaptation of plants to heavy metals toxicity [32,33]. These microbiomes are primarily found in the phyllosphere, rhizosphere, and endosphere (Fig. 1). Revealing the factors responsible for plant microbial assemblage and functions and their interactions can play major roles in enhancing plant growth and sustainable soil health [34].
Fig. 1.
Illustration on the sources of microbial flora representing the plant communities. Adapted from Gupta et al. [35].
The challenges facing global food production are not limited to changes in climatic conditions but also include demographic development. To attain food security sustainably, the maximum exploration of beneficial microbes with the potential to enhance crop productivity and protect plants against pathogens and pests remains a key asset. Hence, the interest in employing microbial inoculants as alternatives to synthetic chemical products in agrarian practices has become critically important.
Nevertheless, limited success has been recorded in exploring potential microorganisms as bioinoculants, which needs to be intensified. Knowledge about the beneficial roles of plant microbes in enhancing sustainable agricultural practices is essential to finding a suitable candidate or viable beneficial microorganism for producing microbial inoculants.
The constituents of root exudates play a crucial role in selecting and enriching microorganisms. This selection depends on the nature of the organic constituents in the root exudates and the ability of the microbes to utilize these exudates as an energy source [36]. Plant microbiomes can actively enhance plant growth through various mechanisms and promote resistance to biotic and abiotic stress. These microbiomes exhibit diverse mechanisms to enhance plant growth, either directly or indirectly. Direct mechanisms include the solubilization of nutrients such as potassium, zinc, and phosphorus, defense against phytopathogens (biocontrol activities), production of siderophores, nitrogen fixation, production of lytic enzymes, and the production of phytohormones such as indole-3-acetic acid, cytokinins, gibberellins, and auxins. Indirect mechanisms encompass the production of hydrogen cyanide, antibiosis, ACC deaminase activity, quorum quenching, competition, induced systemic resistance, and the production of secondary metabolites [37]. Plant nutrients are essential for agricultural sustainability and can be enhanced by applying plant microbiomes in the form of biofertilizers, which increase the availability of nutrients needed for plant nutrition [38,39].
To achieve food security for the growing global population, there is a need to increase the production of healthy crops, which currently rely on chemical fertilizers. However, the use of chemical fertilizers to boost plant productivity can pose detrimental effects on environmental ecology and human health, thus making endophytic fungi alternatives [40,41]. Therefore, the application of beneficial microorganisms is considered an alternative to enhancing soil fertility and promoting sustainable plant productivity.
In agriculture, these microbes are viewed as beneficial and novel biological tools that provide plants with the necessary nutrients for growth. Additionally, microbial consortia with plant growth-promoting, symbiotically compatible traits can be used to replace chemical fertilizers. Numerous research studies have reported the application of endophytic and rhizosphere microbial consortia with plant growth-stimulating traits [42,43].
Studies by Yadav [36] have revealed the presence of various agriculturally important endophytic fungi genera, such as Piriformospora, Aspergillus, Fusarium, and Penicillium, in different food crops. Furthermore, certain host-specific fungal strains have been identified, including Penicillium glabrum in barley, Gibberella zeae in maize, Colletotrichum boninense and C. capsica in soybean, Fusarium verticillioides and Diaporthe endophytica in sugarcane, and Trichoderma atroviride and Talaromyces flavus in wheat [[44], [45], [46], [47], [48], [49]].
3. Nanotechnology linked to endosphere biology: biosynthesis of nanomaterials using microorganisms
There has been commendable progress in the field of nanotechnology, particularly in areas involving the modes of action, synthesis pathways, applications, and characterization techniques of NPs. Chemical and physical methods for synthesizing NPs have proven to be effective, efficient, and time-saving over time. However, the improvement in the production of metal NPs and oxides through these methods has been reported to have ecotoxicological impacts when introduced into the environment. Consequently, the use of microorganisms (fungi, viruses, and bacteria), algae, and yeasts in the "biosynthesis" or "green synthesis" of NPs is gaining popularity. Nanoparticle formation mechanisms vary among microorganisms.
Nanoparticles, generated in microbial systems, often result from.
-
(i)
the trapping/absorption of metal ions on the surface or inside microbial cells, and
-
(ii)
the reduction/depletion of the trapped metal ions to NPs in the presence of enzymes.
In recent years, different microorganisms have been used to synthesize a variety of inorganic NPs with well-defined size, morphology, and chemical composition, and their importance and usage in various notable technological areas have been investigated. Some of their functions include cancer treatment, DNA analysis, biosensors, targeted drug delivery, separation science, gene therapy, enhancing reaction rates, magnetic resonance imaging (MRI), and acting as antibacterial agents [50]. Scientists have recently developed an increasing interest in the interactions between inorganic compounds and biological organisms, and many microorganisms may create inorganic NPs through extracellular or intracellular pathways [51].
The endosphere of a plant is known to harbor numerous microorganisms that hold great importance. These microbes are gaining more attention as many studies have reported that they serve as reservoirs for many notable metabolites of industrial and medical significance [52]. Known as endophytes, these microbes include fungi, bacteria, archaea, and viruses. Endophytic fungi are among the most researched examples of endophytes, and different studies have explored their importance [[53], [54], [55]]. Although scientists globally are exploring ways to overcome current environmental challenges and health implications associated with chemically synthesized NPs, limited studies exist on the application of endophytic fungi in the synthesis of NPs.
Some studies have highlighted the importance of endophytic microbes in the synthesis of NPs using semi-conductive Quantum dots (QDs), which are among the most promising possible biomedical diagnostic materials. Using a biosynthetic technique combined with the microbes’ antibody-combination capabilities, Ray et al. [56] adopted rapid identification of pathogens and employed nanotechnology to sustainably minimize issues related to disease control. Nikolova et al. [57] reported a simple technique for cancer treatment using magnetosomes generated from endophytic microbes. Furthermore, quasi-biosynthesized Ag2Se QDs were promising candidates for in vivo imaging diagnostics as low-toxicity fluorescent tags [58]. Overall, these examples show how biosynthesized nanomaterials may be used in biomedical applications. However, additional in-depth research into the use of biosynthesized NPs from endophytic microbes as nanomedicine has yet to be undertaken. The fungus, Trichoderma viride was used to biosynthesize silver NPs [59]. Extracellularly generated silver NPs utilizing Fusarium oxysporum can be introduced into textile production to prevent or decrease infection with harmful bacteria such as S. aureus, according to a report by Durán et al. [60].
Magnetic particles coupled with biological molecules have also been proposed for use as biological labels, as they are interesting materials for creating assay systems. For the quick and sensitive detection of tiny compounds like environmental contaminants, hormones, and hazardous detergents, competitive chemiluminescence enzyme immunoassays employing antibodies immobilized atop BacMPs were created [61]. The biogenesis of NPs using endophytic microbes is gradually gaining popularity in a variety of fields due to its low cost, ease of use, and environmentally friendly nature. However, to boost their acceptance and application in different sectors, the biochemical, molecular, and cellular mechanisms that mediate the creation of biological NPs using endophytic microbes should be explored in detail.
4. Methods involved in the culturing, extraction, and characterization of isolated endophytic fungi and focus on their nanoparticle production
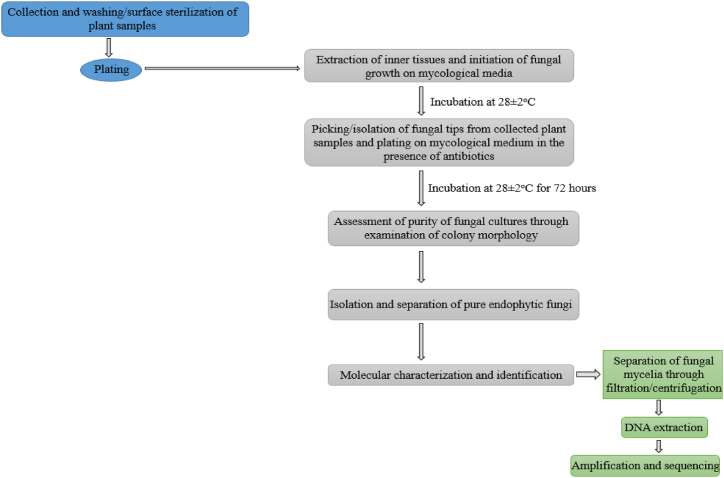
Ubiquitous across the plant kingdom, endophytic fungi play crucial roles in enhancing the growth, yield, and vigor of the host plant [29]. One of the most important advantages of these microbes is their ability to produce various bioactive compounds, which can be processed into novel antioxidant, anti-carcinogenic, and antimicrobial agents [62]. Isolating endophytic fungi using mycological media, such as Potato Dextrose Agar (PDA), Malt Extract Agar (MEA), and Yeast Extract Agar (YEA) and further analyzing them to discover natural products can be beneficial if maximally harnessed in agriculture, medicine, pharmacy, and other industries [62,63]. The plating technique entails isolating endophytic fungi on a Petri dish containing a rich medium, such as PDA. The dehydrated potato infusion and dextrose present in PDA (which acts as a selective medium) stimulate rich and abundant growth while also encouraging the production of pigments and mold sporulation. PDA is also known to aid in the cultivation and differentiation of non-pathogenic and pathogenic fungi [64].
Culturing by plating is an effective method for the detection, enumeration, and isolation of microorganisms from different samples, whether direct or selective. Direct plating involves collecting hyphal spores from a source. This is followed by inoculating them on a fresh plate containing sterilized specific media for fungal isolation, while selective techniques are employed to isolate specific and targeted fungal species from an environment. The selective method is often achieved first by surface sterilization of the fungal host, using selective nutrient temperatures and other conditions, with all techniques focused on enabling the development of the desired species [65]. While direct plating and selective isolation techniques are used for fungi isolation, the most common method for isolating endophytic fungi is the selective plating technique using PDA [66].
The procedure entails sterilization in a 75 % ethanol solution (v/v) for about 1–2 min, after which the host plant is washed 2–3 times with sterilized (deionized) water. Thereafter, a 2 % sodium hypochlorite solution (v/v) is applied for about 1 min for surface sterilization of the tissue, and then the sodium hypochlorite (NaOCl) residue is washed off with sterilized water. The host tissue (stem/root) is then transferred to the PDA plate where fungal growth is initiated. After fungal growth initiation, hyphal tips are collected from the growing colonies and then cultured at about 28 °C in the dark for 72 h in the presence of an antibiotic such as streptomycin to prevent or suppress bacterial growth [67]. Thereafter, an assessment of fungal culture and separation of pure endophytic fungi is done to collect and characterize pure cultures, as illustrated in Fig. 2.
Fig. 2.
Illustration of the isolation procedure of endophytic fungi.
4.1. Extraction of NPs from endophytic fungi
The extraction of NPs from endophytic fungi is a process that leverages the fungi's potential to produce or secrete NPs, offering simplicity and speed. The procedure begins with the isolation of endophytic fungi from healthy plant tissues, such as leaves, stems, or roots. To eliminate contaminants, the plant tissues are surface sterilized by immersion in 70 % ethanol for 1–2 min, followed by washing in 1 % sodium hypochlorite solution for 5–10 min, and then rinsing thoroughly with sterilized distilled water [68].
The sterilized samples are cut into small segments, placed on an agar medium (e.g., PDA or MEA), and incubated at 25–28 °C for 7–14 days to achieve fungal growth. Fungal colonies are then sub-cultured on fresh agar plates to obtain pure cultures. The fungal inoculum is prepared by introducing a spore suspension or fungal mycelium from a pure culture into a liquid mycological growth medium. This inoculated medium is incubated in a shaker incubator at 25–28 °C with constant shaking (150–200 rpm) for 7–14 days, depending on the fungal growth rate. After incubation, the fungal mycelium is separated from the culture broth using sterile filtration or centrifugation to prepare fungal biomass.
To synthesize NPs, the isolated fungi are cultured in suitable liquid or solid media under optimal conditions, such as temperature and pH. After preparation, the fungal biomass is harvested through centrifugation or filtration and washed with sterile distilled water to remove residual growth medium and impurities. The biomass is then dried, either in an oven at low temperatures (30–40 °C) or through air drying, depending on the fungi's sensitivity [69].
Nanoparticle biosynthesis can occur extracellularly or intracellularly. In extracellular synthesis, metal salt solutions are prepared, such as silver nitrate for silver NPs or ferric chloride for iron NPs. These salts are dissolved in sterile distilled water at concentrations ranging from 1:10 to 1:50 (extract: metal solution) and incubated at room temperature or in a shaker to facilitate the reduction of metal ions to NPs, monitored by a color change.
In the extracellular synthesis process, the fungal biomass is suspended in distilled water, and the mixture is centrifuged to obtain the supernatant. The metal salt solution is then added to this supernatant, and the mixture is maintained under optimal growth conditions, including pH, temperature, and metal ion concentration, to enhance NPs yield and size. The formation of NPs is indicated by color changes in the solution. Intracellular synthesis follows a similar process, starting with the preparation of metal salt solutions. The fungal biomass is treated by mixing it directly with the metal salt solution in a suitable reactor or flask, and the mixture is incubated under controlled conditions to facilitate nanoparticle production. In the post-incubation step, the fungal cells are separated from the NPs solution by centrifugation or filtration, and the NPs are purified by washing with distilled water or ethanol to remove any unreacted metal salts [70].
Characterization of the synthesized NPs involves several techniques. UV–visible spectrophotometry confirms NPs formation by measuring absorbance spectra which show absorption at a specific wavelength. Transmission electron microscopy (TEM) and scanning electron microscopy (SEM) visualize the size, morphology, and surface structure. Energy-dispersive X-ray spectroscopy (EDX) analyzes the elemental composition, X-ray diffraction (XRD) determines crystalline structure, and dynamic light scattering (DLS) assesses particle size distribution and stability. Fourier Transform Infrared Spectroscopy (FTIR) identifies functional groups and assesses chemical composition [71]. Optimizing process parameters such as metal salt concentration, pH, temperature, and reaction time can enhance the quality and yield of NPs synthesized from endophytic fungi. Scaling up the process for larger quantities using bioreactors or large fermentation vessels is crucial in industrial applications, where continuous monitoring ensures the consistency and quality of the produced NPs.
4.2. Characterization of endophytic fungi
To identify or characterize endophytic fungi, amplification of ribosomal regions must be performed, followed by DNA sequencing and the BLAST procedure through the National Center for Biotechnology Information (NCBI) platform. Following preliminary identification of isolated endophytic fungi (extracted by the plating methods) based on their microscopic characteristics, confirmation is made by their ITS-rDNA sequences. Primers are usually used in the amplification of the ITS-rDNA sequence [65].
4.3. Nanoparticle-producing potential of endophytic fungi
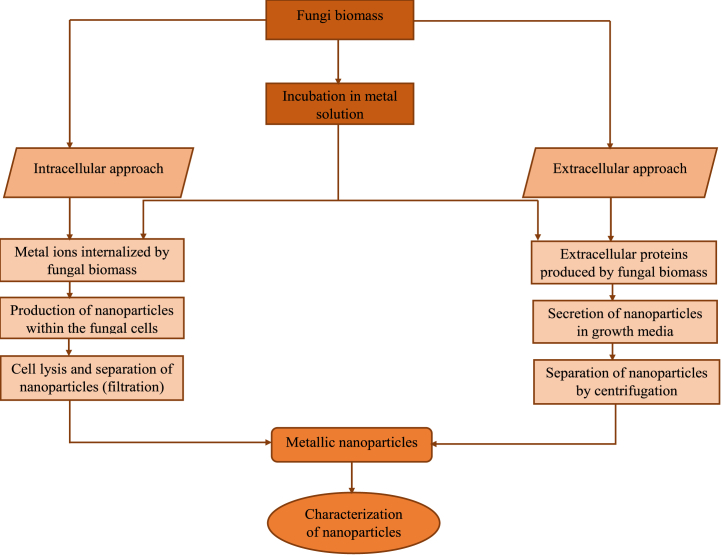
While it's yet to be fully explored, the use of endophytic microbes such as endophytic fungi in the synthesis of NPs is considered a new strategy with bright potential (Fig. 3). Several species of endophytic fungi possess the ability to produce NPs (silver, gold, zinc, magnesium, copper, etc.). For example, Fusarium oxysporum can primarily produce silver NPs using the cell-associated biosynthesis method, while Fusarium solani synthesizes gold NPs [72,73]. Due to their metal uptake ability and the accumulation and tolerance properties of endophytic fungi, these fungi are optimized to synthesize NPs [29]. They produce a greater quantity of enzymes and secondary metabolites necessary for the synthesis and production of NPs [12].
Fig. 3.
Synthesis of NPs from endophytic fungi.
Unlike chemical and physical techniques, using endophytic fungi in nanoparticle synthesis offers numerous benefits regarding cost, energy usage, toxicity, and environmental hazards, as biological agents such as endophytic fungi are inexpensive, eco-friendly, and non-toxic.
According to Messaoudi and Bendahou [1], enzymes and secondary metabolites produced through secondary activities by endophytic fungi can aid in the bioconversion of metal ions into metallic NPs in the production medium.
Endophytic fungi are also considered a superior option for the synthesis of metallic NPs compared to other microbes because.
-
i.
They can be easily isolated from plants [74],
-
ii.
They can secrete a large number of extracellular enzymes and metabolites, which aids the reduction of metal ions into NPs [19],
-
iii.
They are easy to scale up due to their rapid growth [63],
-
iv.
They can tolerate changes in growth factors, such as temperature, pH, and salinity (NaCl), in the culture conditions to synthesize homogenous NPs [75].
Moreover, since synthesis occurs within the cell, downstream processing has been observed to be expensive and difficult [76]. In contrast to intracellular synthesis, extracellular synthesis involves the absorption of metal ions on the cell surface and the depletion of metal ions in the presence of nitrate reductase, an enzyme secreted by endophytic fungi [76].
5. Nanoparticle production with a focus on endophytic fungi
Fungi are capable of producing higher quantities of NPs compared to bacteria because they produce more proteins, which are involved in nanoparticle synthesis. This makes fungi a promising and sustainable source for nanoparticle production [77]. Different fungal species have been reported to produce various NPs. For instance, Penicillium nodositatum, derived from the leaf of R. dumetorium, can produce eco-friendly silver NPs (AgNPs) [78]. Similarly, Setosphaeria sp. BAB-4662, recovered from the stem of Solanum nigrum, produced myco-silver NPs [79]. Baker and Satish [80] and Darwesh et al. [81] reported the production of gold (Au), copper oxide (CuO), and silver (Ag) NPs from Fusarium oxysporum, while Trichothecium sp., Aspergillus fumigatus, and Colletotrichum sp. produced Au, CuO, Ag, and Au NPs, respectively. Other examples of fungi that produce NPs are listed in Table 1.
Table 1.
Nanoparticle production from endophytic fungi and their plant source.
| S/N | Plant source | Fungi | NPs | References |
|---|---|---|---|---|
| 1. | Sorghum bicolor | Trichoderma citrinoviride | TiO2 | [90] |
| 2. | Cleistes fragrans | Fusarium solani | Au | [28] |
| 3. | Commiphora wightii | Cladosporium sp. | Au | [91] |
| 4. | Rauvolfia tetraphylla | Alternaria sp. | Au | [92,93] |
| 6. | Tecomella undulata | Penicillium oxalicum | Fe | [94] |
| 7. | Neoponera foetida | A. nidulans | CoO | [95] |
| 8. | Millingtonia hortensis | Xylaria acuta | ZnO | [96] |
| 7. | Nothapodytes foetida | A. flavus | Zn | [97] |
| 8. | Calendula arvensis | Streptomyces capillispiralis | Cu | [98] |
| 9. | Aegle marmelos | A. terreus | CuO | [99] |
| 10. | Balanites aegyptiaca | Periconium sp. | ZnO | [100] |
| 11. | Avicennia marina, Suaeda monica and Rhizophora mucronata | A. conicus, Penicillium janthinellum, and Phomosis sp. | Ag | [101] |
| 12. | Centella asiatica | A. versicolor | Ag | [102] |
| 13. | Stypandra glauca | A. niger | Ag | [103] |
| 14. | Chonemorpha fragrans | F. solani | Au | [28] |
| 15. | Origanum majorana | A. terreus | Fe3O4 | [104] |
| 16. | Sargassum wightii | C. cladosporioides | Au | [105] |
The mechanism of nanoparticle production in fungi is not well understood, although it can occur through a reduction process [4,78]. This process involves two major steps: firstly, the accumulation of metal ions on the fungal mycelia due to the attraction of positively charged metal ions to the slightly negatively charged cell wall; secondly, the depletion of metal ions by the fungi, leading to nanoparticle formation [82,83]. Specific enzymes, such as reductases, facilitate this process [84]. For example, when silver NPs were produced by Aspergillus flavus, an enzyme named 32-kDa reductase protein synthesized by the fungus was utilized [85]. Despite the advantages of nanoparticle production from organisms, a notable disadvantage is the time consumption involved [86].
Hence, it is necessary to intensify research to discover how to improve nanoparticle production using fungi. It is also essential to intensify research to understand the mechanism of nanoparticle production from fungi. Some endophytic fungi interacting with plants in the Ascomycota, Zygomycota, Mucoromycota, and Basidiomycota classes are known to be mitosporic, and their ecological services have greatly contributed to the survival and development of plants, leading to improved yields [87]. Investigations have revealed their potential for stimulating plant development by suppressing pathogens or improving rhizosphere nutritional conditions, with factors such as soil type, host plant, fungal strain, and compatibility all playing a role [88,89]. These fungi are known to assist plants in overcoming abiotic and biotic stresses and to boost plant nutrition in nutrient-deficient environments.
They offer numerous benefits, such as the ease with which they can be isolated from plants or soil, unlike actinomycetes and bacteria which require specific enrichment procedures for isolation [106]. Most endophytes exhibit a wide range of tolerance to temperature, pH, and salt, which helps in adjusting culture conditions to produce homogenized NPs [107].
Recent interest has increased in employing endophytic fungi for the synthesis of metallic NPs due to their metal absorption, tolerance, and accumulation potentials (Table 2). They secrete high amounts of extracellular enzymes and metabolites, which facilitate the reduction of metal ions to NPs [1]. Compared to other microbes, endophytic fungi show the most promising biological tools for nanoparticle biosynthesis [13]. These organisms are cultured on a large scale, producing NPs of controlled morphology and size. Sundeep, Vijaya Kumar et al. [108] reported the production of silver NPs from Magnifera indica leaves employed in dental restoration as characterized by XRD, PSA, SEM, EDS, and UV–Vis spectroscopy Similarly, Fusarium solani isolated from Chonemorpha fragrans was employed for the biosynthesis of gold (Au) NPs.
Table 2.
Some endophytic fungal isolates biosynthesize metal/metal oxide NPs.
| S/N | Endophytic fungi | NPs | Shape | Size (nm) | Application | References |
|---|---|---|---|---|---|---|
| 1. | A. versicolor | Silver (Ag) | Spherical | 3–40 | Pharmaceutical and biomedical | [102] |
| 2. | A. nidulans | Cobalt oxide (Co3O4) | Spherical | 20–29 | Electrochemical devices, supercapacitors, | [110] |
| 3. | Fusarium solani | Gold (Au) | Spindle | 510–560 | In-vitro anticancer and biomedical | [28] |
| 4. | Alternaria carthami | Zinc oxide (ZnO) | Spherical | 15–45 | Antioxidant, anticancer, and antibacterial | [6] |
| 5. | Penicillium oxalicum | Iron (Fe) | Spherical | 300 | Textile | [29] |
The NPs displayed cytotoxic effects against breast (IC50: 1.3 ± 0.5 μg/mL) and cervical (IC50: 0.8 ± 0.5 μg/mL) cancer cells. The morphology of the synthesized nanomaterial was observed to include mixtures of flower-like, needle, and spindle shapes, with a pink-ruby red coloration. The surface plasmon resonance peaks appeared around 510 and 560 nm [28]. The therapeutic impact of biosynthesized AgNPs mediated by endophytic fungus isolated from Tinospora cordifolia was investigated by Bagur et al. [109]. The NPs produced were spherical and had an average size of 25–35 nm. Hydroxyl groups of phenols, FTIR, and Amide I and II bands of proteins revealed the bioactive metabolites on the surface of AgNPs.
Abdelhakim et al. [111] reported the biogenic synthesis of zinc oxide NPs from the filtrate of Alternaria tenuissima from Erythrophleum fordii. The NPs were spherically shaped, with diameters ranging from 15 to 45 nm, and exhibited antioxidant, anticancer, and antibacterial properties. Bagur et al. [112] also reported the isolation of Exserohilum rostrate, an endophytic fungus from Ocimum tenuiflorum, with the potential to synthesize spherical-shaped silver NPs ranging from 10 to 15 nm, which demonstrated antimicrobial, antioxidant, and anti-inflammatory properties.
6. Biogenic synthesis of endophytic fungi-based NPs
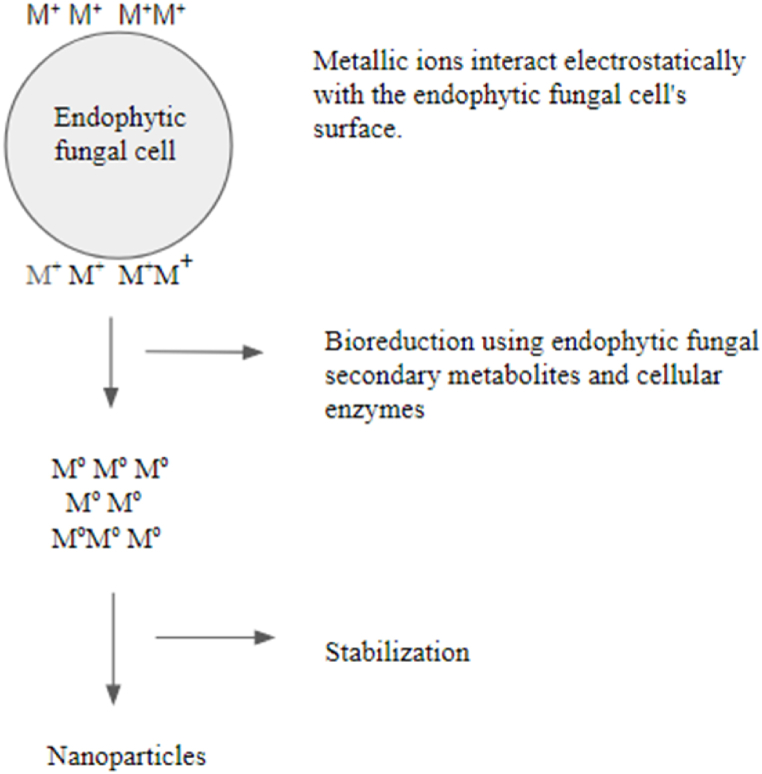
Various physical procedures have been used to synthesize NPs, often requiring significant energy, pressure, and heat. Ethylenediaminetetraacetic acid (EDTA), polyvinyl alcohol (PVA), triethylamine, and thioglycerol are some of the modifiers employed in chemical methods, which may be hazardous and contribute to environmental contamination [113]. These substances may remain in or be bound to the final products of NPs. They are also used as capping and stabilizing agents to control nanoparticle sizes and prevent agglomeration. Recently, there has been increased attention to the green synthesis of metal/metal oxide NPs due to their eco-friendly nature, reduced toxicity, and energy efficiency [114,115]. Plants, fungi, bacteria, and their metabolic by-products can be used in nanoparticle biogenesis as well as function as reducing and stabilizing agents [116,117]. Microbial enzymes and secondary metabolites, both intracellularly and extracellularly, are crucial for the reduction of metal ions into NPs (Fig. 4). For intracellular synthesis, the metal precursor is introduced into the mycelial growth medium and internalized. After synthesis, chemical treatment, filtration, and centrifugation are used to extract NPs and alter the biomass [118]. For extracellular synthesis, the formation of free NPs in dispersion results from introducing the metal precursor to the filtrate, which contains only endophyte biomolecules. There is no procedure for releasing NPs from cells [119].
Fig. 4.
Biogenic synthesis of metal NPs using endophytic fungi.
However, to remove fungal residues and contaminants, nanoparticle dispersion must be filtered using methods such as simple filtering, gel, membrane filtrations, ultracentrifugation, and dialysis. Fungi are relatively more resourceful in nanoparticle biogenic synthesis due to their high accumulation of bioactive metabolites and enhanced production [120].
Despite numerous investigations into biogenic nanoparticle synthesis using fungal extracts, the mechanisms are not yet fully understood. Murillo-Rábago et al. [70] proposed the potential role of fungal media and fungal compounds in stabilizing NPs. Mulay and Deopurkar [121] used Aureobasidium species to investigate the biosynthesis of gold NPs (AuNPs). The production of AuNPs was found to occur in the vacuoles of the three fungal strains, with spherical-shaped AuNPs formed in the presence of reducing sugars and certain proteins capping the NPs.
The non-biodegradable nature and persistence of heavy metals in our environment pose a significant threat to living organisms in both terrestrial and aquatic ecosystems due to their toxicity and bioaccumulation [21]. The presence of inorganic metals is important in the reduction and oxidation of micronutrients. Similarly, heavy metals can affect the survival of living organisms [122].
The potential ecological benefits of endophytic microbes have not been fully realized. Exploring these microbes can aid in the removal or reduction of heavy metals from the soil through various mechanisms, such as the production of enzymes and metabolites [123]. The compounds secreted by these microbes can catalyze biological functions that reduce metal ions in the root environment to nano-sized molecules. This can be achieved by culturing fungal isolates in a heavy-metal-supplemented medium, creating osmotic stress conditions that promote fungal survival through the production of enzymes and metabolites [116].
The synergy between endophytic fungi and plants can enhance the absorption of heavy metals by plants. Utilizing endophytic fungi for the decontamination of waste effluents and environmental pollutants is a promising strategy for pollution control and the restoration of polluted ecosystems [124,125]. Heavy metal absorption by plant roots can be challenging due to slow intrusion mechanisms from the soil, but endophytic microorganisms can facilitate this process, mitigating toxicity effects and soil damage [126]. Endophytic fungi can produce proteins and binding peptides that stimulate the release of effector molecules in response to heavy metal absorption rates in the soil [21]. Additionally, the chemical nature of heavy metals can influence microbial responsiveness to bioremediation. Environmental factors affect the processing, transition, bioavailability, and accessibility of heavy metals to microorganisms. At low pH, heavy metals form free ionic species and protons, which are available for binding. As hydrogen ion concentration increases and positively charges the adsorbent surface, the attraction between the adsorbent and cations decreases, increasing toxicity [21]. However, metabolite compounds and extracellular enzymes from endophytic fungi can facilitate the bioremediation or reduction of toxic metal ions to non-toxic metallic ions [127]. In summary, the mechanisms through which endophytic fungi interact with metal salts to achieve bioremediation can be categorized into two main processes: (i) the ability of endophytic fungi to trap metal ions in the culturing medium and (ii) the production of extracellular enzymes by the endophytic fungi to reduce metal ions.
7. Application of fungal-based NPs for crop productivity and soil fertility
Fungal-based NPs provide a unique and environmentally friendly method for improving agricultural yield and soil fertility [128]. Certain fungi strains, such as Aspergillus have been implicated in the production of silver NPs as evidenced in the study of Wang et al. [129] and Hulikere and Joshi [130] who synthesized silver NPs from Aspergillus sydowii and Cladosporium cladosporioides culture in vitro. NPs, whether metallic (e.g. gold and silver) or non-metallic (e.g. selenium), can provide nutrients, defend against pests, enhance soil structure, stimulate plant growth, and contribute to environmental sustainability [3]. Consequently, they are highly valuable in contemporary agriculture, and these capabilities make them important in contemporary agriculture, as presented below.
7.1. Enhancement of soil health, plant growth, and nutrient uptake
Nanoparticles have antibacterial, antifungal, and antiviral characteristics that can be employed to safeguard agricultural soil against detrimental microbes, hence, creating a safer and more wholesome soil environment for plants and the microbial ecosystem [131]. NPs from endophytic fungi have been found to enhance photosynthesis, reduce oxidative stress, improve drought tolerance and nutrient uptake, promote plant growth and development, increase root and shoot length, and enhance nutrient uptake [55,132,133]. Some fungi, like Aspergillus and Penicillium, have been discovered to have the ability to produce NPs, such as Ag NPs that play a vital role in various physiological processes in plants, ultimately resulting in improved crop yield [[134], [135], [136]].
7.2. Soil remediation
Nanoremediation is a promising approach for the immobilization of inorganic pollutants [128]. By breaking down pollutants, fungal NPs can help in the reclamation of contaminated soil from heavy metals and other toxic contaminants. According to Dhanapal et al. [128], iron NPs (Fe NPs) are highly effective in removing hexavalent chromium contaminants from soil and have a high recovery rate. Due to their capacity to absorb heavy metal contaminants, Fe NPs are also considered excellent in reclaiming soil contaminated with chlorinated organic compounds and heavy metals [137]. In addition to iron NPs, zeolites, iron-oxide, iron sulfide, and phosphate-based NPs have also been recognized for their therapeutic properties in contaminated soil [138]. Aborisade et al. [139] discovered that nanoscale zero-valent iron can reduce the mobilization and build-up of arsenic in plants grown in soil contaminated with metal(loids). NPs can both absorb pollutants and reduce the energy needed for their decomposition [118].
7.3. Pest and disease management
Effective pest and disease management significantly influences agricultural production by enhancing agricultural output and crop yield [140]. Ag NPs and Fe NPs have demonstrated potential in managing arthropod pests, including spider mites, maize earworms, and aphids, as well as controlling insect pests and diseases [141,142]. Research has been conducted on the use of both Ag NPs and silica NPs to treat powdery mildew disease in vegetables such as cucurbits. It has been found that nano-silica can serve as a carrier for validamycin, which aids in the targeted release of pesticides [143,144]. In addition, copper NPs are effective in controlling tomato and cucumber wilt caused by Fusarium species [145,146]. Nano fungicides, nano pesticides, and nano herbicides are widely employed in agriculture to effectively control and manage pests and diseases, hence minimizing reliance on chemical pesticides and fungicides [147].
7.4. Improvement of soil fertility and reduction of drought stress
Fungal NPs can accelerate the breakdown of soil organic matter, resulting in the enrichment of humus and organic compounds leading to enhanced soil fertility and, subsequently, greater crop yield [148,149]. Titanium dioxide, zinc oxide, gold, and silver NPs are being utilized to improve soil health and fertility to promote agricultural yield [150,151]. In addition, studies have shown that silica NPs can help mitigate the effects of drought stress by improving leaf weight, chlorophyll content, and leaf area index [152,153]. Furthermore, these NPs have also been found to enhance plant biomass under conditions of drought stress. Shelke et al. [154] assert that NPs from endophytic fungi offer a viable synergistic approach for improving crop resilience to drought through the enhancement of secondary metabolite production.
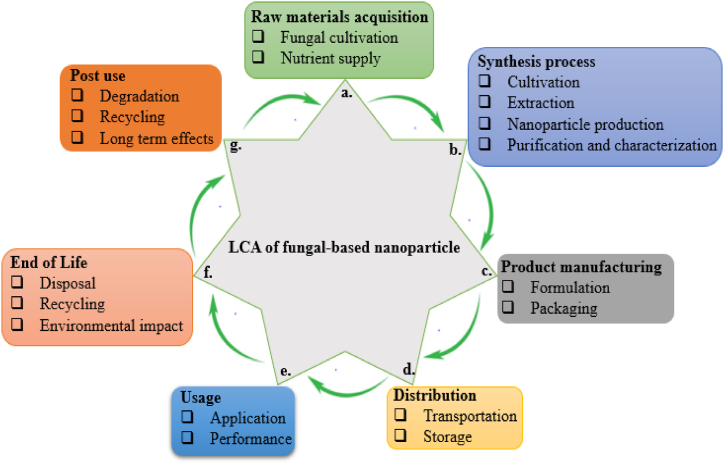
8. Life cycle assessment of fungal-based NPs
The life cycle assessment (LCA) of fungal-based NPs is a comprehensive approach to evaluating their environmental and health impacts across different stages of their life cycle, and this is explained below (Fig. 5).
-
a.
Sourcing of materials: Nutrients and media: This is used to assess the environmental impact of the production and transportation of growth media components, such as sugars, salts, vitamins, and minerals. The sustainability of substrates used for culturing fungi is very important, considering their source (e.g., agricultural waste, synthetic materials) and the environmental impact associated with sourcing them. Chemicals: The environmental footprint of chemicals used for NPs synthesis and purification, including solvents and reducing agents is also important in LCA. Plant materials are critical in assessing the environmental and health impacts associated with the cultivation and transportation of plant materials used for fungal isolation or growth.
-
b.
Culturing, extraction, and synthesis: Culturing is used to evaluate the energy and resources used in growing fungi, including factors like incubation time and temperature control; Extraction and synthesis are considered for the efficient nanoparticle extraction and synthesis processes. This includes evaluating chemical usage, waste production, and energy consumption; Purification and characterization assess the impact of purification processes (e.g., centrifugation, filtration) and characterization methods (e.g., microscopy, spectroscopy) on the environment.
-
c.
Incorporation and packaging: Incorporation examines the processes involved in integrating NPs into products or formulations, including any additional materials or chemicals used; while packaging evaluates the environmental impact of packaging materials and methods.
-
d.
Distribution and storage: Distribution: analyses of the transportation impacts, including fuel consumption, emissions, and packaging used for shipping NPs or nano-products; Storage: Consider the energy required for storage conditions, including temperature and humidity control, and the associated environmental impacts.
-
e.
Application: Assessing the benefits and risks of NPs in agricultural applications, including their potential effects on soil health and crop yield. Evaluation of the potential benefits versus risks in medical applications, with a focus on safety and efficacy. To measure the impacts related to the use of NPs in electronics, including energy consumption and material efficiency. Environmental remediation to measure the effectiveness and environmental impact of NPs in cleaning up pollutants.
-
f.
Recycling, recovery, and waste management: Recycling to explore the feasibility of recycling NPs and the associated impacts; Recovery processes to evaluate the efficient methods for recovering NPs from used products. Waste management to assess the impacts of different disposal methods, such as landfilling or incineration, on the environment and human health.
-
g.
Long-term impacts and recovery: Investigating the potential for recovering materials or reusing NPs from waste and studying how NPs degrade or interact with the environment over time. To assess the potential long-term environmental and health impacts, including accumulation in ecosystems and risks to human exposure.
Fig. 5.
Life cycle assessment (LCA) of fungal-based nanoparticle.
9. Future perspective, challenges, and potential risks
9.1. Future perspective
The future of fungal-based NPs looks promising, with potential applications across various fields, such as medicine, and agriculture. In medicine, these NPs could revolutionize drug delivery systems, thus providing targeted and efficient treatments with minimal side effects. In agriculture, they could enhance crop protection and growth by acting as biopesticides or nutrient carriers, reducing the need for chemical fertilizers and pesticides [76].
The recent findings by Ghareeb et al. [155] highlight the efficacy of the endophytic fungus Aspergillus flavus (ON146363) for real-time applications, particularly in the production of secondary metabolites and volatile organic compounds. These discoveries suggest that endophytic fungi could serve as eco-friendly alternatives to synthetic nematicides. The authors report that these compounds not only promote plant growth and health but also enhance plant defense mechanisms against phytopathogens. Moreover, the ecological significance of endophytic fungi as emerging biorational tools is gaining attention due to their ability to reduce the environmental impact of synthetic chemicals and contribute to sustainable agricultural productivity. Additionally, the use of endophytic fungi capable of producing bioactive compounds, such as NPs, represents a promising strategy for eco-friendly pest management and soil health improvement.
Future research should focus on optimizing the conditions for the application of these fungi to maximize their benefits, including enhancing crop resilience, improving plant-microbe interactions, and integrating them into broader applications across diverse fields. Studying the mechanisms through which these fungi produce beneficial metabolites and bioactive compounds could also provide new insights into their ecological functions. Furthermore, developing bio-formulations using endophytic fungi has the potential to revolutionize eco-friendly agriculture, ensuring sustainable productivity while reducing reliance on harmful synthetic chemicals.
The development of standardized protocols for fungal nanoparticle synthesis, combined with advancements in nanotechnology, will further enhance their applicability and effectiveness. To fully maximize the use of endophytic fungi, optimization of growth media through co-culture fermentation under laboratory conditions for the production of bioactive compounds has been proposed as an efficient technique with promising potential to address challenges associated with endophytic microbiome research in the production of organic compounds [156].
Nevertheless, the continuous research to uncover the full potential of fungi in nanoparticle synthesis is expected to see more industries embracing this green technology. The integration of fungal-based NPs into existing manufacturing processes could lead to the development of hybrid materials with enhanced properties. This could pave the way for innovative products that combine the best of both biological and synthetic fields, offering superior performance with minimal environmental impact [26]. Fungal-based NPs offer a promising future in terms of cost-effectiveness and environmental friendliness for several reasons.
9.1.1. Cost-effective
Fungal-based NPs are advantageous concerning their cost-effectiveness. Traditional NPs synthesis often involves expensive raw materials, high energy consumption, and complex purification processes. In contrast, fungal biosynthesis utilizes inexpensive substrates, such as agricultural waste, and operates under mild conditions. This not only reduces production costs but also offers an opportunity to convert waste into valuable products, adding an economic incentive [8].
The scalability of fungal nanoparticle production is another factor contributing to its cost-effectiveness. Fungi can be cultured on a large scale with minimal infrastructure, making it feasible to produce NPs in industrial quantities [26]. Additionally, the use of fungi in NPs synthesis can reduce the reliance on costly and rare metals, as fungi can synthesize a variety of metallic and non-metallic NPs [157]. As industries seek to lower costs while maintaining high-quality products, fungal-based NPs are poised to become a preferred choice.
9.1.2. Environmental impact and sustainability
The environmental benefits of fungal-based NPs extend beyond their green synthesis process. Unlike conventional NPs, which often pose risks of toxicity and environmental contamination, fungal-based NPs are biodegradable and less likely to accumulate in the environment [158]. This reduces the potential for long-term ecological harm and aligns with global efforts to promote sustainable practices [159]. More also, the use of biogenic NPs synthesis from microorganisms as biostimulants supports the principles of agricultural bioeconomy [160]. By utilizing waste products as substrates, this approach not only mitigates waste disposal issues but also contributes to resource efficiency. The ability of fungi to degrade pollutants and synthesize NPs simultaneously opens new avenues for environmental remediation, thus offering a dual benefit of pollution reduction and NPs production [8]. As environmental regulations become stricter and consumer awareness of sustainability increases, industries will be compelled to adopt greener technologies. Fungal-based NPs, with their low environmental impact, will play a crucial role in this transition, offering a sustainable alternative to conventional nanomaterials.
9.1.3. Sustainability
This bioprospecting of employing fungal-based NPs for sustainability indeed highlights a transformative approach to bridging ecological and technological systems [161]. By leveraging fungi's natural processes for nanoparticle production, this method offers a compelling alternative to traditional manufacturing techniques. Fungi, with their innate ability to undergo bio-mineralization and mycelial growth, present a sustainable pathway to produce biocompatible NPs with potential applications in diverse fields [162]. These NPs could revolutionize industries by facilitating key chemical reactions, such as those involved in waste degradation, energy conversion, and even the creation of new, advanced materials [163]. The sustainable nature of this process, with its reduced carbon footprint and minimal ecological disturbance, underscores its potential as a cornerstone in the transition to a circular economy [164].
Adopting fungal-based nanoparticle synthesis represents more than just a technical advancement—it signals a shift in industrial paradigms. Moving away from synthetic, potentially hazardous materials toward biological systems marks a critical step in aligning industrial processes with ecological principles [165]. This not only enhances resource efficiency but also contributes to a more sustainable and resilient industrial landscape. This approach is well-aligned with global sustainability goals, offering an innovative, eco-friendly solution that minimizes environmental impact while supporting economic growth and technological innovation. It embodies a forward-thinking vision where sustainability and technological progress go hand in hand, paving the way for a future that is both ecologically balanced and technologically advanced.
9.1.4. Biodegradability
The future of fungal-based NPs is indeed promising, especially in terms of environmental sustainability and biodegradability [7]. Fungal species, known for their ability to produce biocompatible and environmentally friendly materials, offer a significant potential to revolutionize various industries [151]. These NPs synthesized through fungal processes, are expected to outperform conventional synthetic NPs in degradation, which can lead to a reduction in harmful waste accumulation in ecosystems. The integration of fungal-based NPs into waste management systems can enhance the breakdown of pollutants, as fungi are adept at metabolizing complex organic substances [118]. The research advancement anticipates the development of more precise methods of engineering these NPs, optimizing their biodegradation rates, and monitoring their effectiveness across diverse environmental conditions.
9.1.5. Ecofriendly synthesis
The use of fungi in this process contributes to a more circular economy by promoting the continuous use of bioresources and reducing overall waste.
Fungal NPs synthesis not only supports environmental sustainability but also offers a pathway to integrate biological processes into industrial practices. This integration can drive innovation and promote eco-conscious practices across various industries. The broader trend towards circular and resource-efficient economic models is well-supported by such advancements, ultimately contributing to more environmentally responsible industrial practices [3].
9.1.6. Efficiency
The efficient application of NPs in drug delivery systems, environmental remediation, and biosensing highlights their significance [166]. The advancement of fungal-based NPs technology promises to unlock new possibilities in fungal genomics studies and synthetic biology through genetic engineering, leading to the development of fungal strains with unique properties and functions [167]. Additionally, integrating fungal-based NPs with other nanomaterials and technologies can result in hybrid systems with synergistic properties, expanding their utility in areas such as electronics, energy storage, and nanomedicine [96].
Research insights into the long-term stability, biocompatibility, and ecological impact of fungal-based NPs will be crucial to ensure their safe and sustainable application. To fully realize their potential, interdisciplinary collaboration among researchers - including mycologists, nanotechnologists, and environmental scientists - will be essential. This collaboration can enhance the efficiency and versatility of fungal-based NPs, positioning them at the forefront of nanotechnology and driving significant advancements in science and industry.
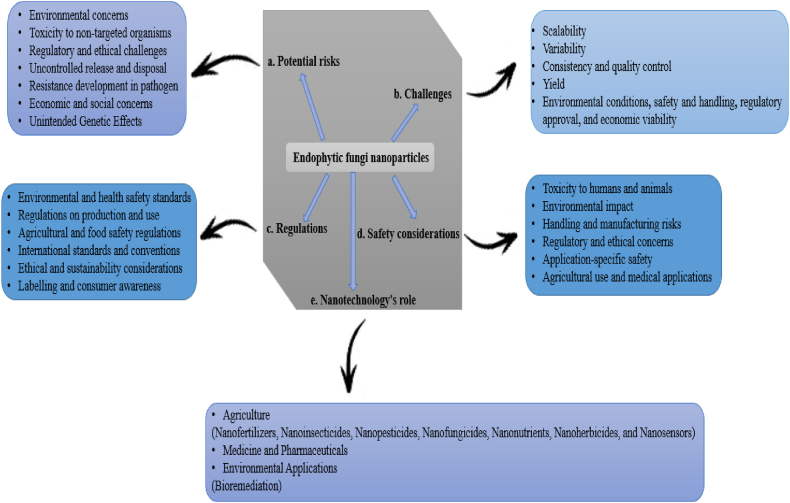
9.2. Challenges and potential risks
Nanoscience offers numerous opportunities across a wide range of fields, including agriculture, medicine, and ecological management [168]. Despite its transformational potential in these areas, the adoption of nanotechnology faces challenges and potential risks that demand careful consideration (Fig. 6). Therefore, gaining insights into the role of nanotechnology in environmental sustainability can enhance understanding of its benefits and facilitate its assessment, particularly about safety and regulatory concerns.
Fig. 6.
The challenges, potential risks, nanotechnology's role, safety considerations, and regulations. a. Environmental concerns: Bioaccumulation in organisms, water, soil, and the food chain; ecosystem disruption; contamination of soil and water bodies; degradation of soil health; reduced plant growth rates; destabilization of ecosystems and loss of biodiversity; unintended consequences on plant-microbe interactions. Toxicity to non-target organisms: Health risks to humans and animals through inhalation or ingestion. Harmful effects may include organ damage, carcinogenicity, cytotoxic and genotoxic effects, oxidative stress, inflammation, and disruption of normal cellular functions. Regulatory and ethical challenges: Ethical concerns, lack of standardized guidelines, and risks of accidental contamination. Uncontrolled release and disposal: Waste accumulation and unintended disposal of waste into water, soil, and the environment. Resistance development in pathogens: The development of resistance in microbes, leads to reduced microbial efficacy and diminished antimicrobial effects. Economic and social concerns: High costs and accessibility challenges, along with potential social resistance. Unintended genetic effects: Alterations and mutations in plant and microbial gene expression. b. Contamination control: Developing stabilization techniques to maintain functional traits, optimizing standard procedures for growth conditions, and implementing safety protocols for adopting NPs from endophytic fungi. c. Regulation of environmental and health safety standards: Risk and ecotoxicological assessments by regulatory agencies such as the World Health Organization (WHO), Food and Agriculture Organization (FAO), Environmental Protection Agency (EPA), and European Chemicals Agency (ECHA). Regulations on production and use should follow Good Manufacturing Practice (GMP), with proper registration and approval. Compliance with agricultural and food safety regulations, including nanoparticle use in agriculture and food additives, should be ensured. International standards and conventions: Adequate measures should be taken by the Organisation for Economic Co-operation and Development (OECD), compliance with World Trade Organization (WTO) trade agreements, and adherence to sanitary and phytosanitary (SPS) measures. Ethical use and sustainability considerations should emphasize research transparency and clear labeling for consumer awareness. d. Toxicity to humans and animals: Monitoring cytotoxic levels, allergic reactions, dosage, and exposure limits is crucial. The environmental impact should be assessed for ecotoxicity, persistence, biodegradability, and interactions with soil microbiomes. Handling and manufacturing risks: Careful monitoring of inhalation hazards, skin and eye irritation, and nanoparticle stability is essential. Regulatory and ethical concerns must also be addressed. e. Nanotechnology in agriculture: Multi-purpose applications of endophytic NPs, including their use as nanofertilizers, nanonutrients, nanoinsecticides, nanopesticides, and nanosensors, as well as in medicine, pharmaceuticals, and environmental applications.
Promisingly, the synthesis of fungal NPs holds great potential for the future, but several challenges need to be addressed to advance this technology [119]. One major issue is variability: the size and shape of NPs synthesized by fungi can vary greatly due to different growth conditions and metal ion concentrations. This variability affects reproducibility, making it difficult to consistently produce NPs with uniform properties. Alterations due to various influences can lead to changes in the stability, toxicity, and reactivity of NPs derived from endophytic fungi. These fungal NPs exhibit size-dependent properties, but achieving a uniform nano-size can be challenging due to variations and uncontrollable conditions inherent in biological systems during nanoparticle production.
Therefore, optimizing the reaction conditions for nanoparticle synthesis by endophytic fungi is crucial. It can significantly improve the uniformity and stability of the NPs, which is essential for ensuring their consistent biological activity. Proper optimization can also help minimize unwanted variability in size and performance. Additionally, such optimization plays a key role in enabling the large-scale production of these NPs. By fine-tuning the synthesis process, it becomes possible to produce NPs with more predictable properties, making them more suitable for practical applications in various industries [169].
Another challenge is scalability: While the technology shows potential, scaling up fungal nanoparticle production can be complex and costly. Processes need to be optimized for industrial production, which often involves navigating intricate and expensive methods. This scalability issue can impact the overall feasibility of large-scale applications [170].
Scaling up nanoparticle biosynthesis from endophytic fungi presents significant challenges, particularly in maintaining consistency in the size and shape of the NPs [171]. Variations often occur during this process, which can affect the overall quality and functionality of the NPs produced. In addition to these challenges, controlling and monitoring nanoparticle production on a larger scale becomes increasingly difficult. Factors, such as growth conditions, nutrient availability, and fungal metabolism may fluctuate, leading to inconsistencies in the final product. Overcoming these obstacles requires the development of precise protocols and advanced monitoring systems to ensure uniformity in nanoparticle biosynthesis. This will enable more reliable and efficient production, especially for industrial applications.
Consistency and quality control: are also critical concerns. Variability in fungal strains, growth conditions, and processing methods can lead to inconsistent results, complicating the reproducibility of NPs. Maintaining consistent quality across different batches is essential for reliable production [172]. Quality control and assurance in nanoparticle production present a significant challenge, particularly due to the genetic and environmental variability of fungal strains sourced from different environments [173]. These variations in growth conditions can impact the consistency and rate of nanoparticle (NP) production. Selecting specific fungal strains with properties conducive to NP production is critical but challenging. Achieving consistent outcomes requires the fine-tuning of growth parameters for the complex reproducibility and characterization of NPs.
The production of metabolites and biomolecules by endophytic fungi offers potential solutions, such as aiding in the purification of NPs and addressing contamination issues, especially in agricultural applications [7]. However, maximizing the production of NPs from these fungi demands the establishment of standardized regulatory guidelines. Such guidelines would be essential for managing the complexity of the process and ensuring quality control, consistency, and safety in nano-products. To address the challenges of consistency and quality control in NP production, it is crucial to optimize growth conditions and standardize protocols. Implementing quality assurance frameworks can also help scale up nanoparticle production from endophytic fungi, contributing to more reliable and safe nano-products for various applications.
Yield: is another factor that influences the effectiveness of fungal NPs synthesis. The yield from fungal sources may be lower compared to other methods, thus affecting cost-effectiveness. Efficiently producing high quantities of NPs remains a significant challenge, as lower yields can impact the economic viability of the technology [17]. Low biomass and inconsistencies can lead to significant variations in yield and growth conditions, as well as in the extraction techniques and substrates used. These variations can negatively impact the large-scale production and reproducibility of NPs, ultimately limiting the overall yield efficiency and the ability to meet demand [174].
To address these challenges, the development and acquisition of specialized bioreactors are essential. Such bioreactors can help maintain consistent growth conditions, optimize substrate use, and improve the overall production process, leading to higher yields. Additionally, the implementation of efficient downstream processing techniques is crucial. These methods can enhance the quality of NPs while maximizing yield, making large-scale production more feasible and cost-effective.
Lastly, environmental conditions, safety and handling, regulatory approval, and economic viability: are crucial considerations. Precise control of environmental conditions, such as temperature, pH, and nutrient availability adds complexity and cost to the process [175]. Additionally, fungal-based processes may introduce biological risks, necessitating careful management. Meeting regulatory standards for various applications and balancing production costs with market demand are essential for long-term success. Moreover, a limited understanding of the exact mechanisms involved in fungal nanoparticle synthesis hampers optimization efforts, highlighting the need for further research into the specific pathways and interactions mediated by enzymes and other biomolecules.
The safe handling of NPs is crucial to mitigating their toxicity, which poses a significant concern for the ecosystem [176]. Despite this, the mechanisms of NP hyper-accumulation in the environment and their entry into food chains, along with the long-term risks they present to human health, are not well understood. These uncertainties highlight the need for special consideration in managing industrial waste disposal containing NPs.
Furthermore, regulatory approval is essential to shift public perception and recognize the importance of nanoscience for economic viability. By establishing clear regulations, the risks associated with NPs can be better managed, ensuring safer applications and reducing environmental and health impacts. To address the challenges of using NPs derived from endophytic fungi effectively and safely, interdisciplinary collaborations are critical. Scientists, researchers, policymakers, and stakeholders must work together to develop guidelines and innovations that promote responsible NP usage while protecting both the environment and human health.
10. Conclusion
Recent research has increasingly focused on NPs derived from endophytic fungi due to their promising potential in various fields. These NPs offer a cost-effective, eco-friendly, and non-toxic alternative to those produced by other methods. Endophytic fungi, which are known for producing bioactive secondary metabolites, can function as natural nano-factories. The biological activities of these fungi often correlate with their NP biosynthesis rates in culture media.
A particularly exciting development is the application of nano-pesticides within myco-nanotechnology. Many agriculturally significant endophytic fungi have bioactive properties that can help control plant pathogens, making this area of research particularly valuable. To advance the field, it is crucial to provide updated information on plant-fungi interactions, the NP-producing potential of these fungi, and their mechanisms and effectiveness in managing plant health. This knowledge will aid in developing green chemistry approaches and expanding the use of NPs from endophytic fungi for human, environmental, and industrial applications.
CRediT authorship contribution statement
Bartholomew Saanu Adeleke: Writing – review & editing, Writing – original draft, Conceptualization. Olumayowa Mary Olowe: Writing – original draft. Modupe Stella Ayilara: Writing – review & editing, Writing – original draft. Oluwaseun Adeyinka Fasusi: Writing – original draft. Oluwadara Pelumi Omotayo: Writing – original draft. Ayomide Emmanuel Fadiji: Writing – original draft. Damian C. Onwudiwe: Writing – review & editing. Olubukola Oluranti Babalola: Writing – review & editing, Writing – original draft, Supervision, Funding acquisition, Conceptualization.
Ethics declarations
Not applicable.
Consent to publication
Not applicable.
Data availability statement
Not applicable.
Funding
This work was supported by the National Research Foundation, South Africa (UID grants: 123634; 132595) awarded to OOB and ICGEB grants CRP/ZAF22-03 to OOB.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Co-author, Prof. Damian C. Onwudiwe is serving in an editorial capacity (Associate Editor) for the Heliyon and was not involved in the editorial review or the decision to publish this article. Therefore, the authors declare that they have no competing interests. If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
Dr. Bartholomew Saanu Adeleke, Dr. Modupe Stella Ayilara, Dr. Adeyinka Oluwaseun Fasusi, Dr. Oluwadara Pelumi Omotayo and Dr. Ayomide Emmanuel Fadiji are grateful to North-West University, South Africa, for the postgraduate bursary. Dr. Bartholomew Saanu Adeleke, Dr. Adeyinka Oluwaseun Fasusi and Dr. Ayomide Emmanuel Fadiji thanked the National Research Foundation and The World Academy of Science for the NRF-TWAS African Renaissance Doctoral scholarship. Dr. Olumayowa Olowe is grateful to the North-West University, South Africa, for the postdoctoral award. Prof. Damian C. Onwudiwe recognizes the National Research Foundation for grants that support research in his research group. Prof. Olubukola Oluranti Babalola acknowledges the National Research Foundation for grants that support work in her research group.
References
- 1.Singh A.V., Shelar A., Rai M., Laux P., Thakur M., Dosnkyi I., Santomauro G., Singh A.K., Luch A., Patil R., Bill J. Harmonization risks and rewards: nano-QSAR for agricultural nanomaterials. J. Agric. Food Chem. 2024;72:835–2852. doi: 10.1021/acs.jafc.3c06466. [DOI] [PubMed] [Google Scholar]
- 2.Adeleke B.S., Akinola S.A., Adedayo A.A., Glick B.R., Babalola O.O. Synergistic relationship of endophyte-nanomaterials to alleviate abiotic stress in plants. Front. Environ. Sci. 2022;10 [Google Scholar]
- 3.Sah M.K., Thakuri B.S., Pant J., Gardas R.L., Bhattarai A. The multifaceted perspective on the role of green synthesis of nanoparticles in promoting a sustainable green economy, Sustainable. Chem. 2024;5:40–59. [Google Scholar]
- 4.Ratan Z.A., Haidere M.F., Nurunnabi M., Shahriar S.M., Ahammad A., Shim Y.Y., Reaney M.J., Cho J.Y. Green chemistry synthesis of silver nanoparticles and their potential anticancer effects. Cancers. 2020;12:855. doi: 10.3390/cancers12040855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saratale R.G., Saratale G.D., Shin H.S., Jacob J.M., Pugazhendhi A., Bhaisare M., Kumar G. New insights on the green synthesis of metallic nanoparticles using plant and waste biomaterials: current knowledge, their agricultural and environmental applications. Environ. Sci. Poll. Res. 2018;25:10164–10183. doi: 10.1007/s11356-017-9912-6. [DOI] [PubMed] [Google Scholar]
- 6.Dadayya M., Subhakar A., Gurubasajar N., Thippeswamy M.G., Veeranna S.H., Basaiah T. Green synthesis of silver nanoparticles from endophytic fungus Alternaria carthami-KUMBMDBT-30. Asian J. Biol. Life Sci. 2023;12:193. [Google Scholar]
- 7.Ahmed S.F., Mofijur M., Rafa N., Chowdhury A.T., Chowdhury S., Nahrin M., Islam A.S., Ong H.C. Green approaches in synthesising nanomaterials for environmental nanobioremediation: technological advancements, applications, benefits and challenges. Enviro.l Res. 2022;204 doi: 10.1016/j.envres.2021.111967. [DOI] [PubMed] [Google Scholar]
- 8.Hassan S., Karaila G.K., Singh P., Meenatchi R., Venkateswaran A.S., Ahmed T., Bansal S., Kamalraj R., Kiran G.S., Selvin J. Implications of fungal nanotechnology for sustainable agriculture-applications and future perspectives. Biocatal. Agric. Biotechnol. 2024;57 [Google Scholar]
- 9.Alewi A.M., Hateet R.R. Biosynthesis and antioxidant of gold nanoparticles by endophyte fungus Aspergillus fumigatus isolated from Sanna surattensis leaves. HIV Nursing. 2022;22:869–873. [Google Scholar]
- 10.Chaudhary P., Chaudhary A., Khati P., Kumar G. Nanotechnology and its role in improvement of crop production for sustainable agriculture. Krishi Sci. 2021;2:22–24. [Google Scholar]
- 11.Joudeh N., Linke D. Nanoparticle classification, physicochemical properties, characterization, and applications: a comprehensive review for biologists. J. Nanobiotechnol. 2022;20:262. doi: 10.1186/s12951-022-01477-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Govindappa M., Lavanya M., Aishwarya P., Pai K., Lunked P., Hemashekhar B., Arpitha B., Ramachandra Y., Raghavendra V.B. Synthesis and characterization of endophytic fungi, Cladosporium perangustum mediated silver nanoparticles and their antioxidant, anticancer and nano-toxicological study. BioNanoSci. 2020;10:928–941. [Google Scholar]
- 13.Gezaf S.A., Hamedo H.A., Ibrahim A.A., Mossa M.I. Mycosynthesis of silver nanoparticles by endophytic fungi: mechanism, characterization techniques and their applications. Microbial Biosystems. 2022;7:48–65. [Google Scholar]
- 14.Gupta P., Rai N., Verma A., Saikia D., Singh S.P., Kumar R., Singh S.K., Kumar D., Gautam V. Green-based approach to synthesize silver nanoparticles using the fungal endophyte Penicillium oxalicum and their antimicrobial, antioxidant, and in vitro anticancer potential. ACS Omega. 2022;7:46653–46673. doi: 10.1021/acsomega.2c05605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Manjunatha D., Megha G.T., Nagaraju S., Akarsh S., Nandish G., Sowmya H.V., Thippeswamy B. Eco-friendly synthesized silver nanoparticles from endophytic fungus Phyllosticta owaniana: KUMBMDBT-32 and evaluation of biomedical properties. Arch. Microbiol. 2023;205:217. doi: 10.1007/s00203-023-03549-1. [DOI] [PubMed] [Google Scholar]
- 16.Nassar A.R.A., Eid A.M., Atta H.M., El Naghy W.S., Fouda A. Exploring the antimicrobial, antioxidant, anticancer, biocompatibility, and larvicidal activities of selenium nanoparticles fabricated by endophytic fungal strain Penicillium verhagenii. Sci. Rep. 2023;13:9054. doi: 10.1038/s41598-023-35360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chauhan A., Anand J., Parkash V., Rai N. Biogenic synthesis: a sustainable approach for nanoparticles synthesis mediated by fungi. Inorganic and Nano-Metal Chem. 2023;53:460–473. [Google Scholar]
- 18.Mourad R.M., Darwesh O.M., Abdel-Hakim A. Enhancing physico-mechanical and antibacterial properties of natural rubber using synthesized Ag-SiO2 nanoparticles. Int. J. Biol. Macromol. 2020;164:3243–3249. doi: 10.1016/j.ijbiomac.2020.08.063. [DOI] [PubMed] [Google Scholar]
- 19.Nassar A.R.A., Atta H.M., Abdel-Rahman M.A., El Naghy W.S., Fouda A. Myco-synthesized copper oxide nanoparticles using harnessing metabolites of endophytic fungal strain Aspergillus terreus: an insight into antibacterial, anti-Candida, biocompatibility, anticancer, and antioxidant activities. BMC Complementary Medic. Therapies. 2023;23:261. doi: 10.1186/s12906-023-04056-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naik B.S. Developments in taxol production through endophytic fungal biotechnology: a review. Oriental Pharm. Experim. Med. 2019;19:1–13. [Google Scholar]
- 21.Sandhu S.S., Shukla H., Shukla S. Biosynthesis of silver nanoparticles by endophytic fungi: its mechanism, characterization techniques and antimicrobial potential. Afr. J. Biotechnol. 2017;16:683–698. [Google Scholar]
- 22.Priyadarshini E., Priyadarshini S.S., Cousins B.G., Pradhan N. Metal-Fungus interaction: review on cellular processes underlying heavy metal detoxification and synthesis of metal nanoparticles. Chemosph. 2021;274 doi: 10.1016/j.chemosphere.2021.129976. [DOI] [PubMed] [Google Scholar]
- 23.Seetharaman P.K., Chandrasekaran R., Periakaruppan R., Gnanasekar S., Sivaperumal S., Abd-Elsalam K.A., Valis M., Kuca K. Functional attributes of myco-synthesized silver nanoparticles from endophytic fungi: a new implication in biomedical applications. Biol. 2021;10:473. doi: 10.3390/biology10060473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hridoy M., Gorapi M., Hossain Z., Noor S., Chowdhury N.S., Rahman M., Muscari I., Masia F., Adorisio S., Delfino D.V. Putative anticancer compounds from plant-derived endophytic fungi: a review. Mol. 2022;27:296. doi: 10.3390/molecules27010296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Adeleke B.S., Babalola O.O. The plant endosphere-hidden treasures: a review of fungal endophytes. Biotechnol. Gen. Eng. Rev. 2021;37:154–177. doi: 10.1080/02648725.2021.1991714. [DOI] [PubMed] [Google Scholar]
- 26.Mostafazade R., Arabi L., Tazik Z., Akaberi M., Bazzaz B.S.F. Green synthesis of gold, copper, zinc, iron, and other metal nanoparticles by fungal endophytes; characterization, and their biological activity: a review. Biocatal. Agric. Biotechnol. 2024;60 [Google Scholar]
- 27.El-Bialy H.A., El-Bastawisy H.S. Elicitors stimulate paclitaxel production by endophytic fungi isolated from ecologically altered Taxus baccata. J. Radiation Res. Appl. Sci. 2020;13:79–87. [Google Scholar]
- 28.Clarance P., Luvankar B., Sales J., Khusro A., Agastian P., Tack J.-C., Al Khulaifi M.M., Al-Shwaiman H.A., Elgorban A.M., Syed A. Green synthesis and characterization of gold nanoparticles using endophytic fungi Fusarium solani and its in-vitro anticancer and biomedical applications. Saudi J. Biol. Sci. 2020;27:706–712. doi: 10.1016/j.sjbs.2019.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mathur P., Saini S., Paul E., Sharma C., Mehtani P. Endophytic fungi mediated synthesis of iron nanoparticles: characterization and application in methylene blue decolorization. Curr. Res. Green Sustainable Chem. 2021;4 [Google Scholar]
- 30.Nasif S.O., Siddique A.B., Siddique A.B., Islam M.M., Hassan O., Deepo D.M., Hossain A. Prospects of endophytic fungi as a natural resource for the sustainability of crop production in the modern era of changing climate. Symbiosis. 2023;89:1–25. [Google Scholar]
- 31.Fadiji A.E., Mortimer P.E., Xu J., Ebenso E.E., Babalola O.O. Biosynthesis of nanoparticles using endophytes: a novel approach for enhancing plant growth and sustainable agriculture. Sustainability. 2022;14 [Google Scholar]
- 32.Grabka R., d'Entremont T.W., Adams S.J., Walker A.K., Tanney J.B., Abbasi P.A., Ali S. Fungal endophytes and their role in agricultural plant protection against pests and pathogens. Plants. 2022;11:384. doi: 10.3390/plants11030384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domka A.M., Rozpaądek P., Turnau K. Are fungal endophytes merely mycorrhizal copycats? The role of fungal endophytes in the adaptation of plants to metal toxicity. Front. Microbiol. 2019;10:371. doi: 10.3389/fmicb.2019.00371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Adeleke B.S., Fadiji A.E., Ayilara M.S., Igiehon O.N., Nwachukwu B.C., Babalola O.O. Strategies to enhance the use of endophytes as bioinoculants in agriculture. Hortic. 2022;8:498. [Google Scholar]
- 35.Gupta R., Anand G., Gaur R., Yadav D. Plant–microbiome interactions for sustainable agriculture: a review. Physiol. Mol. Biol. Plants. 2021;27:165–179. doi: 10.1007/s12298-021-00927-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yadav A.N. Biodiversity and biotechnological applications of host-specific endophytic fungi for sustainable agriculture and allied sectors. Acta Sci. Microbiol. 2018;1:1–5. [Google Scholar]
- 37.Ahsan S., Injamum-Ul-Hoque M., Das A.K., Rahman M.M., Mollah M.M.I., Paul N.C., Choi H.W. Plant–Entomopathogenic fungi interaction: recent progress and future prospects on endophytism-mediated growth promotion and biocontrol. Plants. 2024;13:1420. doi: 10.3390/plants13101420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yadav A., Verma P., Kumar R., Kumar V., Kumar K. Current applications and future prospects of eco-friendly microbes. EU Voice. 2017;3:21–22. [Google Scholar]
- 39.Yadav A.N., Kumar R., Kumar S., Kumar V., Sugitha T., Singh B., Chauhan V., Dhaliwal H.S., Saxena A.K. Beneficial microbiomes: biodiversity and potential biotechnological applications for sustainable agriculture and human health. J. Appl. Biol. Biotechnol. 2017;5:45–57. [Google Scholar]
- 40.Fu S.F., Balasubramanian V.K., Chen C.L., Tran T.T., Muthuramalingam J.B., Chou J.Y. The phosphate-solubilising fungi in sustainable agriculture: unleashing the potential of fungal biofertilisers for plant growth. Folia Microbiol. 2024;69:697–712. doi: 10.1007/s12223-024-01181-0. [DOI] [PubMed] [Google Scholar]
- 41.Díaz-Urbano M., Goicoechea N., Velasco P., Poveda J. Development of agricultural bio-inoculants based on mycorrhizal fungi and endophytic filamentous fungi: Co-inoculants for improve plant-physiological responses in sustainable agriculture. Biol. Contrl. 2023;182 [Google Scholar]
- 42.Khan Z., Kandel S.L., Ramos D.N., Ettl G.J., Kim S.H., Doty S.L. Increased biomass of nursery-grown Douglas-fir seedlings upon inoculation with diazotrophic endophytic consortia. Forests. 2015;6:3582–3593. [Google Scholar]
- 43.Bilal S., Shahzad R., Khan A.L., Kang S.M., Imran Q.M., Al-Harrasi A., Yun B.W., Lee I.J. Endophytic microbial consortia of phytohormones-producing fungus Paecilomyces formosus LHL10 and bacteria Sphingomonas sp. LK11 to Glycine max L. regulates physio-hormonal changes to attenuate aluminum and zinc stresses. Front. Plant Sci. 2018;9:1273. doi: 10.3389/fpls.2018.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Khan A.L., Hamayun M., Kim Y.H., Kang S.M., Lee I.J. Ameliorative symbiosis of endophyte (Penicillium funiculosum LHL06) under salt stress elevated plant growth of Glycine max L. Plant Physiol. Biochem. 2011;49:852–861. doi: 10.1016/j.plaphy.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 45.de Souza Leite T., Cnossen-Fassoni A., Pereira O.L., Mizubuti E.S.G., de Araújo E.F., de Queiroz M.V. Novel and highly diverse fungal endophytes in soybean revealed by the consortium of two different techniques. J. Microbiol. 2023;51:56–69. doi: 10.1007/s12275-013-2356-x. [DOI] [PubMed] [Google Scholar]
- 46.Waqas M., Khan A.L., Hamayun M., Shahzad R., Kang S.M., Kim J.G., Lee I.J. Endophytic fungi promote plant growth and mitigate the adverse effects of stem rot: an example of Penicillium citrinum and Aspergillus terreus. J. Plant Interact. 2015;10:280–287. [Google Scholar]
- 47.Deshmukh S., Hückelhoven R., Schäfer P., Imani J., Sharma M., Weiss M., Waller F., Kogel K.H. The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proceed. Nat. Acad. Sci. 2006;103:18450–18457. doi: 10.1073/pnas.0605697103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Köhl J., Lombaers C., Moretti A., Bandyopadhyay R., Somma S., Kastelein P. Analysis of microbial taxonomical groups present in maize stalks suppressive to colonization by toxigenic Fusarium spp.: a strategy for the identification of potential antagonists. Biol. Contr. 2015;83:20–28. [Google Scholar]
- 49.Colla G., Rouphael Y., Bonini P., Cardarelli M. Coating seeds with endophytic fungi enhances growth, nutrient uptake, yield and grain quality of winter wheat. Int. J. Plant Prod. 2015;9:171–190. [Google Scholar]
- 50.Hulkoti N.I., Taranath T. Biosynthesis of nanoparticles using microbes—a review. Colloids Surfaces. 2014;121:474–483. doi: 10.1016/j.colsurfb.2014.05.027. [DOI] [PubMed] [Google Scholar]
- 51.Tauseef A., Hisam F., Hussain T., Caruso A., Hussain K., Châtel A., Chénais B. Nanomicrobiology: emerging trends in microbial synthesis of nanomaterials and their applications. J. Cluster Sci. 2023;34:639–664. [Google Scholar]
- 52.Adeleke B.S., Babalola O.O. Pharmacological potential of fungal endophytes associated with medicinal plants: a review. J. Fungi. 2021;7:147. doi: 10.3390/jof7020147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Qin X., Xu J., An X., Yang J., Wang Y., Dou M., Wang M., Huang J., Fu Y. Insight of endophytic fungi promoting the growth and development of woody plants. Crit. Rev. Biotechnol. 2024;44:78–99. doi: 10.1080/07388551.2022.2129579. [DOI] [PubMed] [Google Scholar]
- 54.Bhunjun C.S., Phukhamsakda C., Hyde K.D., McKenzie E.H., Saxena R.K., Li Q. Do all fungi have ancestors with endophytic lifestyles? Fungal Divers. 2024;125:73–98. [Google Scholar]
- 55.Attia M.S., Salem M.S., Abdelaziz A.M. Endophytic fungi Aspergillus spp. reduce Fusarial wilt disease severity, enhance growth, metabolism and stimulate the plant defense system in pepper plants. Biomass Conversion Biorefinery. 2024;14:16603–16613. [Google Scholar]
- 56.Ray M.K., Mishra A.K., Mohanta Y.K., Mahanta S., Chakrabartty I., Kungwani N.A., Avula S.K., Panda J., Pudake R.N. Nanotechnology as a promising tool against phytopathogens: a futuristic approach to agriculture. Agric. For. 2023;13:1856. [Google Scholar]
- 57.Nikolova M.P., Joshi P.B., Chavali M.S. Updates on biogenic metallic and metal oxide nanoparticles: therapy, drug delivery and cytotoxicity. Pharm. Times. 2023;15:1650. doi: 10.3390/pharmaceutics15061650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gu Y.P., Cui R., Zhang Z.L., Xie Z.-X., Pang D.W. Ultrasmall near-infrared Ag2Se quantum dots with tunable fluorescence for in vivo imaging. J. Amer. Chem. Society. 2012;134:79–82. doi: 10.1021/ja2089553. [DOI] [PubMed] [Google Scholar]
- 59.Fayaz A.M., Balaji K., Girilal M., Yadav R., Kalaichelvan P.T., Venketesan R. Biogenic synthesis of silver nanoparticles and their synergistic effect with antibiotics: a study against gram-positive and gram-negative bacteria. Nanomed. Nanotechnol. Biol. Med. 2010;6:103–109. doi: 10.1016/j.nano.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 60.Durán N., Marcato P.D., De Souza G.I., Alves O.L., Esposito E. Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J. Biomed. Nanotechnol. 2007;3:203–208. [Google Scholar]
- 61.Li X., Xu H., Chen Z.S., Chen G. Biosynthesis of nanoparticles by microorganisms and their applications. J. Nanomater. 2011;2011 [Google Scholar]
- 62.Sopalun K., Laosripaiboon W., Wachirachaikarn A., Iamtham S. Biological potential and chemical composition of bioactive compounds from endophytic fungi associated with Thai mangrove plants. South Afr. J. Bot. 2021;141:66–76. [Google Scholar]
- 63.Mishra V.K., Passari A.K., Chandra P., Leo V.V., Kumar B., Uthandi S., Thankappan S., Gupta V.K., Singh B.P. Determination and production of antimicrobial compounds by Aspergillus clavatonanicus strain MJ31, an endophytic fungus from Mirabilis jalapa L. using UPLC-ESI-MS/MS and TD-GC-MS analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0186234. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 64.Joshi M., Srivastava R., Sharma A., Prakash A. Isolation and characterization of Fusarium oxysporum, a wilt causing fungus, for its pathogenic and non-pathogenic nature in tomato (Solanum lycopersicum) J. Appl. Nat. Sci. 2013;5:108–117. [Google Scholar]
- 65.Xiao Y., Zhang Z., Liang W., Gao B., Wang Y., Chang J., Zhu D. Endophytic fungi from Dongxiang wild rice (Oryza rufipogon Griff.) show diverse catalytic potential for converting glycyrrhizin. 3 Biotech. 2022;12:79. doi: 10.1007/s13205-022-03138-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Afzal I., Shinwari Z.K., Iqrar I. Selective isolation and characterization of agriculturally beneficial endophytic bacteria from wild hemp using canola. Pak. J. Bot. 2015;47:1999–2008. [Google Scholar]
- 67.Zhang Y., Wan Y., Tie Y., Zhang B., Wang R., Wang G. Isolation and characterization of endophytic fungi from purslane and the effects of isolates on the growth of the host. Adv. Microbiol. 2019;9:438–453. [Google Scholar]
- 68.Deepak H., Virk V. Optimization of surface sterilization method for the isolation of endophytic fungi associated with Curcuma longa L. and their antibacterial activity. J. Adv. Biotechnol. Exper. Therapeutics. 2022;5:334–346. [Google Scholar]
- 69.Ben Mefteh F., Frikha F., Daoud A., Chenari Bouket A., Luptakova L., Alenezi F.N., Al-Anzi B.S., Oszako T., Gharsallah N., Belbahri L. Response surface methodology optimization of an acidic protease produced by Penicillium bilaiae isolate TDPEF30, a newly recovered endophytic fungus from healthy roots of date palm trees (Phoenix dactylifera L.) Microorganisms. 2019;7:74. doi: 10.3390/microorganisms7030074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Murillo-Rábago E.I., Vilchis-Nestor A.R., Juarez-Moreno K., Garcia-Marin L.E., Quester K., Castro-Longoria E. Optimized synthesis of small and stable silver nanoparticles using intracellular and extracellular components of fungi: an alternative for bacterial inhibition. Antibiotics. 2022;11:800. doi: 10.3390/antibiotics11060800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Banala R.R., Nagati V.B., Karnati P.R. Green synthesis and characterization of Carica papaya leaf extract coated silver nanoparticles through X-ray diffraction, electron microscopy and evaluation of bactericidal properties. Saudi J. Biol. Sci. 2015;22:637–644. doi: 10.1016/j.sjbs.2015.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zahoor M., Nazir N., Iftikhar M., Naz S., Zekker I., Burlakovs J., Uddin F., Kamran A.W., Kallistova A., Pimenov N. A review on silver nanoparticles: classification, various methods of synthesis, and their potential roles in biomedical applications and water treatment. Water. 2021;13:2216. [Google Scholar]
- 73.Gopinath K., Arumugam A. Extracellular mycosynthesis of gold nanoparticles using Fusarium solani. Appl. Nanosc. 2014;4:657–662. [Google Scholar]
- 74.Kharkwal A.C., Joshi H., Shandilya C., Dabral S., Kumar N., Varma A. Isolation and characterization of a newly discovered plant growth-promoting endophytic fungal strain from the genus Talaromyces. Sci. Reports. 2024;14:6022. doi: 10.1038/s41598-024-54687-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kumar V., Ahluwalia V., Saran S., Kumar J., Patel A.K., Singhania R.R. Recent developments on solid-state fermentation for production of microbial secondary metabolites: challenges and solutions. Biores. Technol. 2021;323 doi: 10.1016/j.biortech.2020.124566. [DOI] [PubMed] [Google Scholar]
- 76.Bhardwaj K., Sharma A., Tejwan N., Bhardwaj S., Bhardwaj P., Nepovimova E., Shami A., Kalia A., Kumar A., Abd-Elsalam K.A. Pleurotus macrofungi-assisted nanoparticle synthesis and its potential applications: a review. J. Fungi. 2020;6:351. doi: 10.3390/jof6040351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rajput K., Raghuvanshi S., Bhatt A., Rai S.K., Agrawal P.K. A review on synthesis silver nano-particles. Int. J. Curr. Microbiol. Appl. Sci. 2017;6:1513–1528. [Google Scholar]
- 78.Manjunath Hullikere M., Joshi C.G., Raju N. Biogenic synthesis of silver nano particles using endophytic fungi Penicillium nodositatum and its antibacterial activity. J. Chem. Pharm. Res. 2014;6:112–117. [Google Scholar]
- 79.Akther T., Khan M.S., Srinivasan H. A facile and rapid method for green synthesis of silver myco nanoparticles using endophytic. Int. J. Nano Dimension. 2018;9:435–441. [Google Scholar]
- 80.Baker S., Satish S. Endophytes: toward a vision in synthesis of nanoparticle for future therapeutic agents. Int. J. Bio-Inorg. Hybd. Nanomat. 2012;1:67–77. [Google Scholar]
- 81.Darwesh O.M., Li H., Matter I.A. Nano-bioremediation of textile industry wastewater using immobilized CuO-NPs myco-synthesized by a novel Cu-resistant Fusarium oxysporum OSF18. Environ. Sci. Poll. Res. 2023;30:6694–16706. doi: 10.1007/s11356-022-23360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mukherjee P., Ahmad A., Mandal D., Senapati S., Sainkar S.R., Khan M.I., Parishcha R., Ajaykumar P., Alam M., Kumar R. Fungus-mediated synthesis of silver nanoparticles and their immobilization in the mycelial matrix: a novel biological approach to nanoparticle synthesis. Nano Lett. 2021;1:515–519. [Google Scholar]
- 83.Siddiqi K.S., Husen A. Fabrication of metal nanoparticles from fungi and metal salts: scope and application. Nanoscale Res. Lett. 2016;11:98. doi: 10.1186/s11671-016-1311-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Dhillon G.S., Brar S.K., Kaur S., Verma M. Green approach for nanoparticle biosynthesis by fungi: current trends and applications. Crit. Rev. Biotechnol. 2012;32:49–73. doi: 10.3109/07388551.2010.550568. [DOI] [PubMed] [Google Scholar]
- 85.Jain N., Bhargava A., Majumdar S., Tarafdar J., Panwar J. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale. 2011;3:635–641. doi: 10.1039/c0nr00656d. [DOI] [PubMed] [Google Scholar]
- 86.Tashi T., Gupta N.V., Mbuya V.B. Silver nanoparticles: synthesis, mechanism of antimicrobial action, characterization, medical applications, and toxicity effects. J. Chem. Pharm. Res. 2016;8:526–537. [Google Scholar]
- 87.Hiruma K., Kobae Y., Toju H. Beneficial associations between Brassicaceae plants and fungal endophytes under nutrient-limiting conditions: evolutionary origins and host–symbiont molecular mechanisms. Curr. Opinion Plant Biol. 2018;44:145–154. doi: 10.1016/j.pbi.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 88.Acuña‐Rodríguez I.S., Newsham K.K., Gundel P.E., Torres‐Díaz C., Molina‐Montenegro M.A. Functional roles of microbial symbionts in plant cold tolerance. Ecol. Lett. 2020;23:1034–1048. doi: 10.1111/ele.13502. [DOI] [PubMed] [Google Scholar]
- 89.Hassan S.E.D. Plant growth-promoting activities for bacterial and fungal endophytes isolated from medicinal plant of Teucrium polium L. J. Adv. Res. 2017;8:687–695. doi: 10.1016/j.jare.2017.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Arya S., Sonawane H., Math S., Tambade P., Chaskar M., Shinde D. Biogenic titanium nanoparticles (TiO2NPs) from Tricoderma citrinoviride extract: synthesis, characterization and antibacterial activity against extremely drug-resistant Pseudomonas aeruginosa. Int. Nano Lett. 2021;11:35–42. [Google Scholar]
- 91.Munawer U., Raghavendra V.B., Ningaraju S., Krishna K.L., Ghosh A.R., Melappa G., Pugazhendhi A. Biofabrication of gold nanoparticles mediated by the endophytic Cladosporium species: photodegradation, in vitro anticancer activity and in vivo antitumor studies. Int. J. Pharm. 2020;588 doi: 10.1016/j.ijpharm.2020.119729. [DOI] [PubMed] [Google Scholar]
- 92.Verma V.C., Singh S.K., Solanki R., Prakash S. Biofabrication of anisotropic gold nanotriangles using extract of endophytic Aspergillus clavatus as a dual functional reductant and stabilizer. Nanoscale Res. Lett. 2011;6:16. doi: 10.1007/s11671-010-9743-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hemashekhar B., Chandrappa C., Govindappa M., Chandrashekar N. Endophytic fungus Alternaria spp isolated from Rauvolfia tetraphylla root arbitrate synthesis of gold nanoparticles and evaluation of their antibacterial, antioxidant and antimitotic activities. Adv. Natural Sci.: Nanosci. Nanotechnol. 2019;10 [Google Scholar]
- 94.Mathur P., Saini S., Paul E., Sharma C., Mehtani P. Endophytic fungi mediated synthesis of iron nanoparticles: characterization and application in methylene blue decolorization. Curr. Res. Green Sustainable Chem. 2021;4 [Google Scholar]
- 95.Vijayanandan A.S., Balakrishnan R.M. Biosynthesis of cobalt oxide nanoparticles using endophytic fungus Aspergillus nidulans. J. Environ. Manag. 2018;218:442–450. doi: 10.1016/j.jenvman.2018.04.032. [DOI] [PubMed] [Google Scholar]
- 96.Sumanth B., Lakshmeesha T.R., Ansari M.A., Alzohairy M.A., Udayashankar A.C., Shobha B., Niranjana S.R., Srinivas C., Almatroudi A. Mycogenic synthesis of extracellular zinc oxide nanoparticles from Xylaria acuta and its nanoantibiotic potential. Int. J. Nanomed. 2020;15:8519. doi: 10.2147/IJN.S271743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Uddandarao P. ZnS semiconductor quantum dots production by an endophytic fungus Aspergillus flavus. Materials Sci. Eng. 2016;207:26–32. [Google Scholar]
- 98.Hassan S.E.D., Salem S.S., Fouda A., Awad M.A., El-Gamal M.S., Abdo A.M. New approach for antimicrobial activity and bio-control of various pathogens by biosynthesized copper nanoparticles using endophytic actinomycetes. J. Rad. Res. Appl. Sci. 2018;11:262–270. [Google Scholar]
- 99.Mani V.M., Kalaivani S., Sabarathinam S., Vasuki M., Soundari A.J.P.G., Das M.A., Elfasakhany A., Pugazhendhi A. Copper oxide nanoparticles synthesized from an endophytic fungus Aspergillus terreus: bioactivity and anti-cancer evaluations. Environ. Res. 2021;201 doi: 10.1016/j.envres.2021.111502. [DOI] [PubMed] [Google Scholar]
- 100.Ganesan V., Hariram M., Vivekanandhan S., Muthuramkumar S. Periconium sp. (endophytic fungi) extract mediated sol-gel synthesis of ZnO nanoparticles for antimicrobial and antioxidant applications. Materials Sci. Semiconductor Process. 2020;105 [Google Scholar]
- 101.Bharathidasan R., Panneerselvam A. Biosynthesis and characterization of silver nanoparticles using endophytic fungi Aspergillus concius, Penicillium janthinellum and Phomosis sp. Int. J. Pharm. Sci. Res. 2012;3:3163. [Google Scholar]
- 102.Netala V.R., Bethu M.S., Pushpalatha B., Baki V.B., Aishwarya S., Rao J.V., Tartte V. Biogenesis of silver nanoparticles using endophytic fungus Pestalotiopsis microspora and evaluation of their antioxidant and anticancer activities. Int. J. Nanomed. 2016;11:5683. doi: 10.2147/IJN.S112857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hemashekhar B., Chandrappa C., Govindappa M., Chandrasekhar N., Nagaraju G., Ramachandra Y. Green synthesis of silver nanoparticles from endophytic fungus Aspergillus niger isolated from Simarouba glauca leaf and its Antibacterial and Antioxidant activity. Inter J Eng Res Appl. 2017;7:17–24. [Google Scholar]
- 104.Mousa S.A., El-Sayed E.S.R., Mohamed S.S., Abo El-Seoud M.A., Elmehlawy A.A., Abdou D.A. Novel mycosynthesis of Co3O4, CuO, Fe3O4, NiO, and ZnO nanoparticles by the endophytic Aspergillus terreus and evaluation of their antioxidant and antimicrobial activities. Appl. Microbiol. Biotechnol. 2021;105:741–753. doi: 10.1007/s00253-020-11046-4. [DOI] [PubMed] [Google Scholar]
- 105.Joshi C.G., Danagoudar A., Poyya J., Kudva A.K., Dhananjaya B. Biogenic synthesis of gold nanoparticles by marine endophytic fungus-Cladosporium cladosporioides isolated from seaweed and evaluation of their antioxidant and antimicrobial properties. Process Biochem. 2017;63:137–144. [Google Scholar]
- 106.Compant S., Samad A., Faist H., Sessitsch A. A review on the plant microbiome: ecology, functions, and emerging trends in microbial application. J. Adv. Res. 2019;19:29–37. doi: 10.1016/j.jare.2019.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Alkahtani M.D., Fouda A., Attia K.A., Al-Otaibi F., Eid A.M., Ewais E.E.D., Hijri M., St-Arnaud M., Hassan S.E.D., Khan N. Isolation and characterization of plant growth promoting endophytic bacteria from desert plants and their application as bioinoculants for sustainable agriculture. Agronomy. 2020;10:1325. [Google Scholar]
- 108.Sundeep D., Vijaya Kumar T., Rao P.S., Ravikumar R., Gopala Krishna A. Green synthesis and characterization of Ag nanoparticles from Mangifera indica leaves for dental restoration and antibacterial applications. Progress Biomat. 2017;6:57–66. doi: 10.1007/s40204-017-0067-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Bagur H., Medidi R.S., Somu P., Choudhury P.J., Karua C.S., Guttula P.K., Melappa G., Poojari C.C. Endophyte fungal isolate mediated biogenic synthesis and evaluation of biomedical applications of silver nanoparticles. Materials Technol. 2022;37:167–178. [Google Scholar]
- 110.Venkateswarulu N., Shameer S., Bramhachari P., Basha S.T., Nagaraju C., Vijaya T. Isolation and characterization of plumbagin (5-hydroxyl-2-methylnaptalene-1, 4-dione) producing endophytic fungi Cladosporium delicatulum from endemic medicinal plants. Biotechnol. Rep. 2018;20 doi: 10.1016/j.btre.2018.e00282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abdelhakim H.K., El‐Sayed E., Rashidi F.B. Biosynthesis of zinc oxide nanoparticles with antimicrobial, anticancer, antioxidant and photocatalytic activities by the endophytic Alternaria tenuissima. J. Appl. Microbiol. 2020;128:1634–1646. doi: 10.1111/jam.14581. [DOI] [PubMed] [Google Scholar]
- 112.Bagur H., Poojari C.C., Melappa G., Rangappa R., Chandrasekhar N., Somu P. Biogenically synthesized silver nanoparticles using endophyte fungal extract of Ocimum tenuiflorum and evaluation of biomedical properties. J. Cluster Sci. 2020;31:1241–1255. [Google Scholar]
- 113.Ahmed T., Noman M., Manzoor N., Ali S., Rizwan M., Ijaz M., Allemailem K.S., BinShaya A.S., Alhumaydhi F.A., Li B. Recent advances in nanoparticles associated ecological harms and their biodegradation: global environmental safety from nano-invaders. J. Environ. Chem. Eng. 2021;9 [Google Scholar]
- 114.Bhattacharjee N., Som I., Saha R., Mondal S. A critical review on novel eco-friendly green approach to synthesize zinc oxide nanoparticles for photocatalytic degradation of water pollutants. Int. J. Environ. Analyt. Chem. 2024;104:489–516. [Google Scholar]
- 115.Ogbonna C., Kavaz D. Green synthesis of hybrids of zinc oxide, titanium oxide, and calcium oxide nanoparticles from Foeniculum vulgare: an assessment of biological activity. Chem. Eng. Comm. 2024;211:1072–1098. [Google Scholar]
- 116.Hussain A., Shah M., Hamayun M., Qadir M., Iqbal A. Heavy metal tolerant endophytic fungi Aspergillus welwitschiae improves growth, ceasing metal uptake and strengthening antioxidant system in Glycine max L. Environ. Sci. Poll. Res. 2022;29:15501–15515. doi: 10.1007/s11356-021-16640-1. [DOI] [PubMed] [Google Scholar]
- 117.Hussain M.I., Muscolo A., Farooq M., Ahmad W. Sustainable use and management of non-conventional water resources for rehabilitation of marginal lands in arid and semiarid environments. Agric. Water Management. 2019;221:462–476. [Google Scholar]
- 118.Rajput V.D., Minkina T., Upadhyay S.K., Kumari A., Ranjan A., Mandzhieva S., Sushkova S., Singh R.K., Verma K.K. Nanotechnology in the restoration of polluted soil. Nanomaterials. 2022;12:769. doi: 10.3390/nano12050769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhao X., Zhou L., Riaz Rajoka M.S., Yan L., Jiang C., Shao D., Zhu J., Shi J., Huang Q., Yang H. Fungal silver nanoparticles: synthesis, application and challenges. Crit. Rev. Biotechnol. 2018;38:817–835. doi: 10.1080/07388551.2017.1414141. [DOI] [PubMed] [Google Scholar]
- 120.Soni V., Raizada P., Singh P., Cuong H.N., Rangabhashiyam S., Saini A., Saini R.V., Van Le Q., Nadda A.K., Le T.T. Sustainable and green trends in using plant extracts for the synthesis of biogenic metal nanoparticles toward environmental and pharmaceutical advances: a review. Environ. Res. 2021;202 doi: 10.1016/j.envres.2021.111622. [DOI] [PubMed] [Google Scholar]
- 121.Mulay Y., Deopurkar R. Purification, characterization of amylase from indigenously isolated Aureobasidium pullulans Cau 19 and its bioconjugates with gold nanoparticles. Appl. Biochem. Biotechnol. 2018;184:644–658. doi: 10.1007/s12010-017-2575-4. [DOI] [PubMed] [Google Scholar]
- 122.Yunusa M.A., Igwe E.C., Mofoluke A.O. Heavy metals contamination of water and fish-a review. Fudma J. Sci. 2023;7:110–118. [Google Scholar]
- 123.Yao S., Zhou B. Enhancing phytoremediation of cadmium and arsenic in alkaline soil by Miscanthus sinensis: a study on the synergistic effect of endophytic fungi and biochar. Sci. Total Environ. 2024;923 doi: 10.1016/j.scitotenv.2024.171458. [DOI] [PubMed] [Google Scholar]
- 124.Chaurasia P.K., Nagraj, Sharma N., Kumari S., Yadav M., Singh S., Mani A., Yadava S., Bharati S.L. Fungal assisted bio‐treatment of environmental pollutants with comprehensive emphasis on noxious heavy metals: recent updates. Biotechnol. Bioeng. 2023;120:57–81. doi: 10.1002/bit.28268. [DOI] [PubMed] [Google Scholar]
- 125.Nandy S., Das T., Tudu C.K., Pandey D.K., Dey A., Ray P. Fungal endophytes: futuristic tool in recent research area of phytoremediation. South Afr. J. Bot. 2020;134:285–295. [Google Scholar]
- 126.Chaudhary P., Xu M., Ahamad L., Chaudhary A., Kumar G., Adeleke B.S., Verma K.K., Hu D.M., Širić I., Kumar P. Application of synthetic consortia for improvement of soil fertility, pollution remediation, and agricultural productivity: a review. Agronomy. 2023;13:643. [Google Scholar]
- 127.Dwivedi S.K. Fungi mediated detoxification of heavy metals: insights on mechanisms, influencing factors and recent developments. J. Water Process Eng. 2023;53 [Google Scholar]
- 128.Dhanapal A.R., Thiruvengadam M., Vairavanathan J., Venkidasamy B., Easwaran M., Ghorbanpour M. Nanotechnology approaches for the remediation of agricultural polluted soils. ACS Omega. 2024;9:13522–13533. doi: 10.1021/acsomega.3c09776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wang D., Xue B., Wang L., Zhang Y., Liu L., Zhou Y. Fungus-mediated green synthesis of nano-silver using Aspergillus sydowii and its antifungal/antiproliferative activities. Sci. Rep. 2021;11 doi: 10.1038/s41598-021-89854-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Hulikere M.M., Joshi C.G. Characterization, antioxidant and antimicrobial activity of silver nanoparticles synthesized using marine endophytic fungus-Cladosporium cladosporioides. Process Biochem. 2019;82:199–204. [Google Scholar]
- 131.Dutta P., Kumari A., Mahanta M., Upamanya G.K., Heisnam P., Borua S., Kaman P.K., Mishra A., Mallik M., Muthukrishnan G. Nanotechnological approaches for management of soil-borne plant pathogens. Front. Plant Sci. 2023;14 doi: 10.3389/fpls.2023.1136233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Amin M.A.A., Abu-Elsaoud A.M., Nowwar A.I., Abdelwahab A.T., Awad M.A., Hassan S.E.D., Boufahja F., Fouda A., Elkelish A. Green synthesis of magnesium oxide nanoparticles using endophytic fungal strain to improve the growth, metabolic activities, yield traits, and phenolic compounds content of Nigella sativa L. Green Process. Synth. 2024;13 [Google Scholar]
- 133.Liaquat F., Kim H.S., Kim S., Alyan A., Shah I.H., Oh N.H., Chung H., Choe H., Yun H.Y. Application of Trichoderma spp. combined with iron oxide nanoparticles improves the physiochemical response of Arabidopsis thaliana under drought. J. Plant Biol. 2023;66:395–405. [Google Scholar]
- 134.Yahia W.A., Elkhawaga M.A., Mahfouz A.Y., Attia M.S. Achieving green synthesis of silver nanoparticles by Aspergillus ustus ON076464 for improving Immune response and vegetative growth of pepper plant towards wilt disease caused by Fusarium oxysporum, Egypt. J. Chem. 2023;66:131–151. [Google Scholar]
- 135.Zakariya N.A., Majeed S., Jusof W.H.W. Investigation of antioxidant and antibacterial activity of iron oxide nanoparticles (IONPS) synthesized from the aqueous extract of Penicillium spp. Sensors Int. 2022;3 [Google Scholar]
- 136.Yassin M.A., Elgorban A.M., El-Samawaty A.E.R.M., Almunqedhi B.M. Biosynthesis of silver nanoparticles using Penicillium verrucosum and analysis of their antifungal activity. Saudi J. Biol. Sci. 2021;28:2123–2127. doi: 10.1016/j.sjbs.2021.01.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Shipley H.J., Engates K.E., Guettner A.M. Study of iron oxide nanoparticles in soil for remediation of arsenic. J. Nanoparticle Res. 2011;13:2387–2397. [Google Scholar]
- 138.Liu R., Lal R. Nanoenhanced materials for reclamation of mine lands and other degraded soils: a review. J. Nanotechnol. 2012;2012 [Google Scholar]
- 139.Aborisade M.A., Geng H., Oba B.T., Kumar A., Ndudi E.A., Battamo A.Y., Liu J., Chen D., Okimiji O.P., Ojekunle O.Z., Yang Y. Remediation of soil polluted with Pb and Cd and alleviation of oxidative stress in Brassica rapa plant using nanoscale zerovalent iron supported with coconut-husk biochar. J. Plant Physiol. 2023;287 doi: 10.1016/j.jplph.2023.154023. [DOI] [PubMed] [Google Scholar]
- 140.Ghormade V., Deshpande M.V., Paknikar K.M. Perspectives for nano-biotechnology enabled protection and nutrition of plants. Biotechnol. Adv. 2011;29:792–803. doi: 10.1016/j.biotechadv.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 141.Malik M.A., Wani A.H., Bhat M.Y., Siddiqui S., Alamri S.A., Alrumman S.A. Fungal-mediated synthesis of silver nanoparticles: a novel strategy for plant disease management. Front. Microbiol. 2024;15 doi: 10.3389/fmicb.2024.1399331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.adr B.K.T.N., Alzubaidy M.H.J. Biosynthesis and characterization of nano-iron by Beuveria bassiana fungus. Valeol.: Int. J. Med. Anthropol. Bioethics. 2024;2:63–69. [Google Scholar]
- 143.Virk V., Deepak H., Taneja K., Srivastava R., Giri S. Amelioration in nanobiosensors for the control of plant diseases: current status and future challenges. Front. Nanotechnol. 2024;6 [Google Scholar]
- 144.Nasif S.O., Nuruzzaman M., Naidu R. Porous silica nanocarriers: advances in structural orientation and modification to develop sustainable pesticide delivery systems. ACS Agric. Sci. Technol. 2024;4:144–172. [Google Scholar]
- 145.Al-Qaissi A.R., Fouzi S.H., AL-Baytay A.J. Characterization of biologically synthesized copper nanoparticles by Aspergillus carbonarius and evaluation of their activity in reducing the pathogenic stress of tomatoes infected with Fusarium oxysporum. Tikrit J. Agric. Sci. 2024;24:161–177. [Google Scholar]
- 146.Li X., Liu J. Soil amendments with nanoparticles control cucumber wilt caused by Fusarium oxysporium. South Afr. J. Bot. 2024;168:384–393. [Google Scholar]
- 147.Mubeen I., Mfarrej M.F.B., Razaq Z., Iqbal S., Naqvi S.A.H., Hakim F., Mosa W.F., Moustafa M., Fang Y., Li B. Nanopesticides in comparison with agrochemicals: outlook and future prospects for sustainable agriculture. Plant Physiol. Biochem. 2023;198 doi: 10.1016/j.plaphy.2023.107670. [DOI] [PubMed] [Google Scholar]
- 148.Kumar N., Samota S.R., Venkatesh K., Tripathi S.C. Global trends in use of nano-fertilizers for crop production: advantages and constraints–A review. Soil Tillage Res. 2023;228 [Google Scholar]
- 149.Strekalovskaya E.I., Perfileva A.I., Krutovsky K.V. Zinc Oxide Nanoparticles in the “Soil–bacterial community–plant” system: impact on the stability of soil ecosystems. Agronomy. 2024;14:1588. [Google Scholar]
- 150.Kashisaz M., Enayatizamir N., Fu P., Eslahi M. Co-application of beneficial microorganisms and nanoparticles to improve wheat growth in infected Fusarium culmorum soil. Appl. Soil Ecol. 2024;203 [Google Scholar]
- 151.Balusamy S.R., Joshi A.S., Perumalsamy H., Mijakovic I., Singh P. Advancing sustainable agriculture: a critical review of smart and eco-friendly nanomaterial applications. J. Nanobiotechnol. 2023;21:372. doi: 10.1186/s12951-023-02135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ahmed M., Hassen F., Qadeer U., Aslam M.A. Silicon application and drought tolerance mechanism of sorghum. Afr. J. Agric. Res. 2011;6:594–607. [Google Scholar]
- 153.Mahmoud A.W., Rashad H.M., Esmail S.E., Alsamadany H., Abdeldaym E.A. Application of silicon, zinc, and zeolite nanoparticles—a tool to enhance drought stress tolerance in coriander plants for better growth performance and productivity. Plants. 2023;12:2838. doi: 10.3390/plants12152838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Shelke D.B., Islam N.F., Chambhare M.R., Sonawane H.B., Patowary R., Prasad R., Sarma H. Enhancing secondary metabolites and alleviating environmental stress in crops with mycogenic nanoparticles: a comprehensive review. Biocatal. Agric. Biotechnol. 2023;52 [Google Scholar]
- 155.Ghareeb R.Y., Jaremko M., Abdelsalam N.R., Abdelhamid M.M., El-Argawy E., Ghozlan M.H. Biocontrol potential of endophytic fungi against phytopathogenic nematodes on potato (Solanum tuberosum L.) Sci. Reports. 2024;14 doi: 10.1038/s41598-024-64056-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Gupta A., Meshram V., Gupta M., Goyal S., Qureshi K.A., Jaremko M., Shukla K.K. Fungal endophytes: microfactories of novel bioactive compounds with therapeutic interventions; A comprehensive review on the biotechnological developments in the field of fungal endophytic biology over the last decade. Biomol. 2023;13:1038. doi: 10.3390/biom13071038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Imade E.E., Ajiboye T.O., Fadiji A.E., Onwudiwe D.C., Babalola O.O. Green synthesis of zinc oxide nanoparticles using plantain peel extracts and the evaluation of their antibacterial activity. Sci. Afr. 2022;16 [Google Scholar]
- 158.Sudheer S., Bai R.G., Muthoosamy K., Tuvikene R., Gupta V.K., Manickam S. Biosustainable production of nanoparticles via mycogenesis for biotechnological applications: a critical review. Environ. Res. 2022;204 doi: 10.1016/j.envres.2021.111963. [DOI] [PubMed] [Google Scholar]
- 159.Akanmu A.O., Babalola O.O., Venturi V., Ayilara M.S., Adeleke B.S., Amoo A.E., Sobowale A.A., Fadiji A.E., Glick B.R. Plant disease management: leveraging on the plant-microbe-soil interface in the biorational use of organic amendments. Front. Plant Sci. 2021;12:1590. doi: 10.3389/fpls.2021.700507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Tolisano C., Del Buono D. Biobased: biostimulants and biogenic nanoparticles enter the scene. Sci. Total Environ. 2023;885 doi: 10.1016/j.scitotenv.2023.163912. [DOI] [PubMed] [Google Scholar]
- 161.Chadha U., Selvaraj S.K., Ashokan H., Hariharan S.P., Mathew Paul V., Venkatarangan V., Paramasivam V. Complex nanomaterials in catalysis for chemically significant applications: from synthesis and hydrocarbon processing to renewable energy applications. Adv. Materials Sci. Eng. 2022;2022 [Google Scholar]
- 162.Abd El Hamid D.K., Desouky E.M., AbdEllatif S., Abed N., Mahfouz A.Y. Green synthesis and characterization of titanium dioxide nanoparticles by Aspergillus niger DS22 and its potential application in medical fields. Egypt. J. Bot., Le. 2024;64:629–653. [Google Scholar]
- 163.Darwesh O.M., Al-Balakocy N.G., Ghanem A., Matter I.A. Application of microalgal-ZnO-NPs for reusing polyester/cotton blended fabric wastes after modification by cellulases enzymes. Waste Disposal Sustainable Energy. 2023;5:471–482. [Google Scholar]
- 164.Devi R., Verma R., Dhalaria R., Kumar A., Kumar D., Puri S., Thakur M., Chauhan S., Chauhan P.P., Nepovimova E. A systematic review on endophytic fungi and its role in the commercial applications. Planta. 2023;257:70. doi: 10.1007/s00425-023-04087-2. [DOI] [PubMed] [Google Scholar]
- 165.Kuppan N., Padman M., Mahadeva M., Srinivasan S., Devarajan R. A comprehensive review of sustainable bioremediation techniques: eco friendly solutions for waste and pollution management. Waste Management Bulletin. 2024;2:154–171. [Google Scholar]
- 166.El-Shanshoury A.R., Darwesh O.M., Sabae S.Z., Awadallah O.A., Hassan S.H. Bio-manufacturing of selenium nanoparticles by Bacillus subtilis isolated from Qarun Lake and evaluation their activity for water remediation. Biointerface Res. Appl. Chem. 2020;10:5834–5842. [Google Scholar]
- 167.Manzoor M.A., Shah I.H., Sabir I.A., Ahmad A., Albasher G., Dar A.A., Altaf M.A., Shakoor A. Environmental sustainable: biogenic copper oxide nanoparticles as nano-pesticides for investigating bioactivities against phytopathogens. Environ. Res. 2023;231 doi: 10.1016/j.envres.2023.115941. [DOI] [PubMed] [Google Scholar]
- 168.Osman A.I., Zhang Y., Farghali M., Rashwan A.K., Eltaweil A.S., Abd El-Monaem E.M., Mohamed I.M., Badr M.M., Ihara I., Rooney D.W. Synthesis of green nanoparticles for energy, biomedical, environmental, agricultural, and food applications: a review. Environ. Chem. Lett. 2024;22:841–887. [Google Scholar]
- 169.Xue B., He D., Gao S., Wang D., Yokoyama K., Wang L. Biosynthesis of silver nanoparticles by the fungus Arthroderma fulvum and its antifungal activity against genera of Candida, Aspergillus and Fusarium. Int. J. Nanomed. 2016;11:1899–1906. doi: 10.2147/IJN.S98339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rami M.R., Meskini M., Sharafabad B.E. Fungal-mediated nanoparticles for industrial applications: synthesis and mechanism of action. J. Infection Public Health. 2024;17 doi: 10.1016/j.jiph.2024.102536. [DOI] [PubMed] [Google Scholar]
- 171.El-Moslamy S.H., Elnouby M.S., Rezk A.H., El-Fakharany E.M. Scaling-up strategies for controllable biosynthetic ZnO NPs using cell free-extract of endophytic Streptomyces albus: characterization, statistical optimization, and biomedical activities evaluation. Sci. Reports. 2023;13:3200. doi: 10.1038/s41598-023-29757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Atiq M., Naeem I., Sahi S.T., Rajput N.A., Haider E., Usman M., Shahbaz H., Fatima K., Arif E., Qayyum A. Nanoparticles: a safe way towards fungal diseases. Arch. Phytopathol. Plant Protection. 2020;53:781–792. [Google Scholar]
- 173.Bellanthudawa B., Nawalage N., Handapangoda H., Suvendran S., Wijayasenarathne K., Rathnasuriya M., Wickramasinghe P., Aberathna A., Tennakoon A., Perera I. A perspective on biodegradable and non-biodegradable nanoparticles in industrial sectors: applications, challenges, and future prospects. Nanotechnol. Environ. Eng. 2023;8:975–1013. [Google Scholar]
- 174.Herdiana Y., Wathoni N., Shamsuddin S., Muchtaridi M. Scale-up polymeric-based nanoparticles drug delivery systems: development and challenges. OpenNano. 2022;7 [Google Scholar]
- 175.Bamal D., Singh A., Chaudhary G., Kumar M., Singh M., Rani N., Mundlia P., Sehrawat A.R. Silver nanoparticles biosynthesis, characterization, antimicrobial activities, applications, cytotoxicity and safety issues: an updated review. Nanomaterials. 2021;11:2086. doi: 10.3390/nano11082086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Tran T.K., Nguyen M.K., Lin C., Hoang T.D., Nguyen T.C., Lone A.M., Khedulkar A.P., Gaballah M.S., Singh J., Chung W.J. Review on fate, transport, toxicity and health risk of nanoparticles in natural ecosystems: emerging challenges in the modern age and solutions toward a sustainable environment. Sci. Total Environ. 2024;912 doi: 10.1016/j.scitotenv.2023.169331. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.