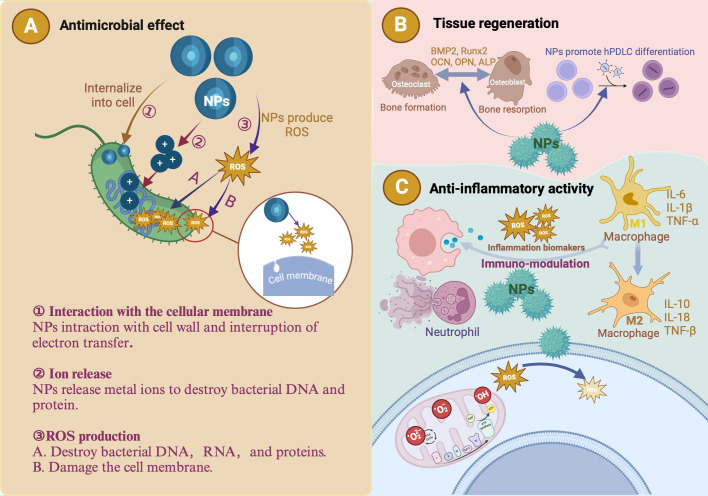
Figure 3.
The antibacterial, anti-inflammatory and tissue regeneration mechanisms of nanoparticles (NPs). (A) The antibacterial effects of metallic NPs are attributed to three primary mechanisms: ROS production, ion release, and interaction with the cellular membrane. ROS generated by these NPs degrade essential cellular components (DNA, RNA, and proteins), effectively killing periodontal pathogens. Due to their small size, metallic NPs can penetrate the peptidoglycan layer and damage bacterial cells. The metal ions released by NPs are toxic to bacterial DNA and proteins. (B) Introduction of metallic NPs results in a microenvironment characterized by low levels of inflammatory cytokines and high levels of reparative cytokines, such as bone morphogenetic protein-2 (BMP-2), runt-related transcription factor 2 (Runx2), osteocalcin (OCN), osteopontin (OPN), and alkaline phosphatase (ALP). This favorable environment promotes human periodontal ligament cell (hPDLC) differentiation and periodontal tissue regeneration and halts the progression of periodontitis. (C) NPs exhibit antioxidant properties that help regulate ROS. Metallic NPs reduce inflammation by regulating cytokines and macrophage polarization.