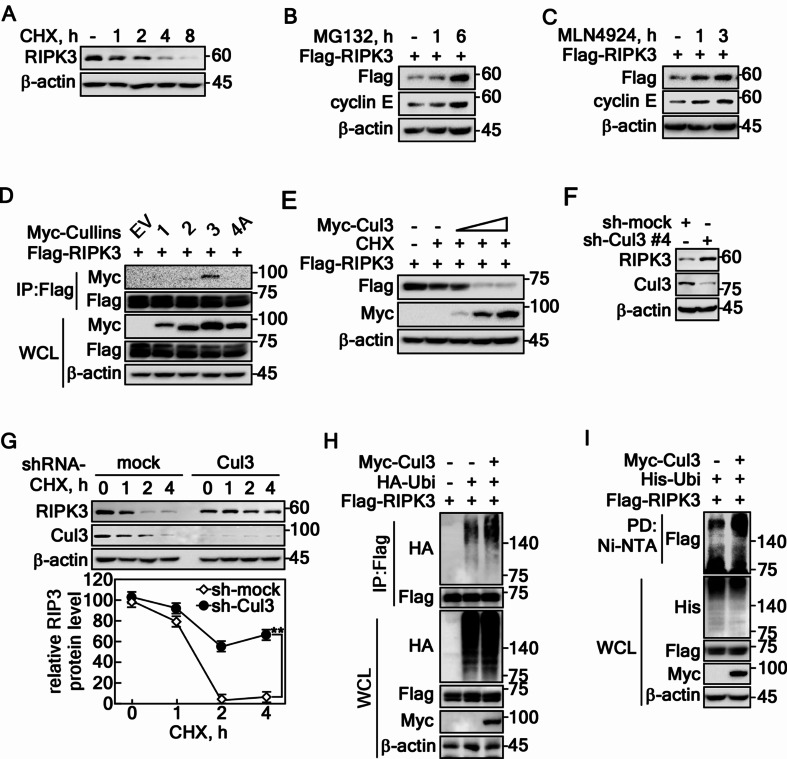
Fig. 1.
Cullin3-composed E3 ligase regulates RIPK3 stability. A RIPK3 protein degradation was evaluated by cycloheximide (10 µg/ml) in HT-29 cells in a time-dependent manner. B The recovery of RIPK3 was confirmed in HeLa cells treated with MG132 (10 µM) in a time-dependent manner. C Cullin inhibition results in an increased level of RIPK3 protein in HeLa cells. D The specific interaction between RIPK3 and Cul3 was confirmed in HEK293T cells. E Cul3 overexpression suppresses the RIPK3 protein levels in a dose-dependent manner in HEK293T cells. F Increased RIPK3 protein level was confirmed by the knockdown of Cul3 using sh-Cul3 in HT-29 cells. G RIPK3 half-life was prolonged by sh-Cullin 3 in HT-29 cells. The error bars obtained from three independent experiments indicate the SEM. ** p < 0.01 (Student t-test). H IP confirmed the Cul3-mediated increased RIPK3 ubiquitination of RIPK3 in HEK293T cells. I Cul3-mediated increased RIPK3 ubiquitination was confirmed by the pulldown of ubiquitin in HEK293T cells. A-I β-actin was used as an internal control for an equal protein loading