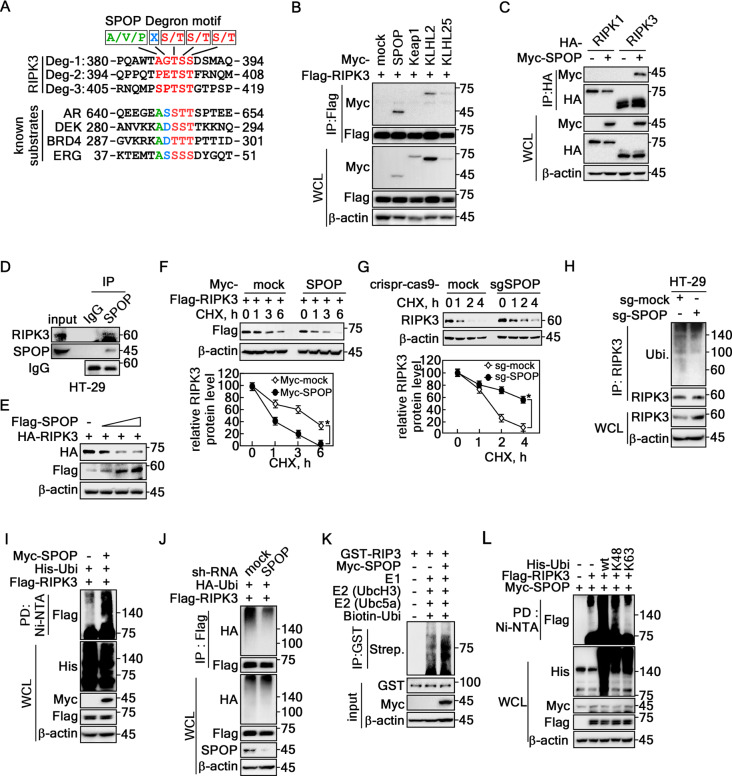
Fig. 2.
SPOP-induced RIPK3 K48 ubiquitination reduces RIPK3 half-life. A Amino acid sequence analysis of the RIPK3 showed putative degron motifs for the SPOP, A/V/P-X-S/T-S/T-S/T. B Screening of RIPK3 interaction with cullin 3 composing E3 ligases by IP in HEK293T cells. C Specific interaction between RIPK3 and SPOP was confirmed by IP in HEK293T cells. D IP confirmed the endogenous interaction of RIPK3 with SPOP in HT-29 cells. E RIPK3 protein levels were decreased by SPOP expression in a dose-dependent manner in HEK293T cells. F Top panels, RIPK3 half-life was shortened by the coexpression of SPOP in HeLa cells. Graphs, Normalized RIPK3 band intensities corresponding β-actin intensity. G Top panels, Knockout SPOP by Crispr-Cas9-SPOP extended RIPK3 half-life in HT-29 cells. Graphs, Normalized RIPK3 band intensities corresponding β-actin intensity. H RIPK3 ubiquitination in the endogenous levels was decreased in Crispr-Cas9-SPOP knockout HT-29 cells. I SPOP overexpression induced RIPK3 ubiquitination in HEK293T cells. J SPOP knockdown abolished RIPK3 ubiquitination in HEK293T cells. K In vitro RIPK3 ubiquitination assay. L Confirmation of the K48-ubiquitination of RIPK3 was mediated by SPOP in HEK293T cells. B-C and E-L β-actin was used as an internal control for an equal protein loading. F and G The error bars from three independent experiments indicate the SEM. * p < 0.05 (Student t-test).