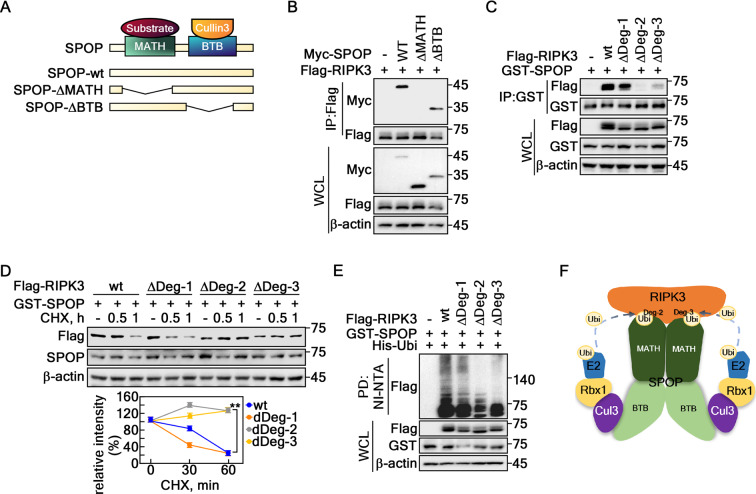
Fig. 3.
MATH domain of SPOP interacts with two degron motifs at the linker domain. A Schematic maps of the SPOP deletion mutants, SPOP-wt, SPOP-ΔMATH, and SPOP-ΔBTB. B MATH domain of SPOP interacted with RIPK3 in HEK293T cells. C SPOP recognized Deg-2 and -3 of RIPK3. ΔDeg-1, deleted aa 385–389; ΔDeg-2, deleted aa 399–403; and ΔDeg-3, deleted aa 409–413 by IP in HEK293T cells. D The prolonged half-life of RIPK3-ΔDeg-2 and -3. Upper panels, Confirmation of the prolonged half-life of RIPK3-ΔDeg-2 and -3 in HeLa cells. Graphs, The band intensities of RIPK3 by normalization using the β-actin band intensity. Densitometric computer program: NIH Image J (Ver. 1.42). The error bars from three independent experiments indicate the SEM. ** p < 0.01 (Student t-test). E SPOP-mediated RIPK3 ubiquitination was decreased in RIPK3-ΔDeg-2 and -3 detected by a Ni-NTA pulldown assay in HEK293T cells. F A proposed binding model of SPOP E3 ligase complex and RIPK3. A and C-E. β-actin was used as an internal control for an equal protein loading