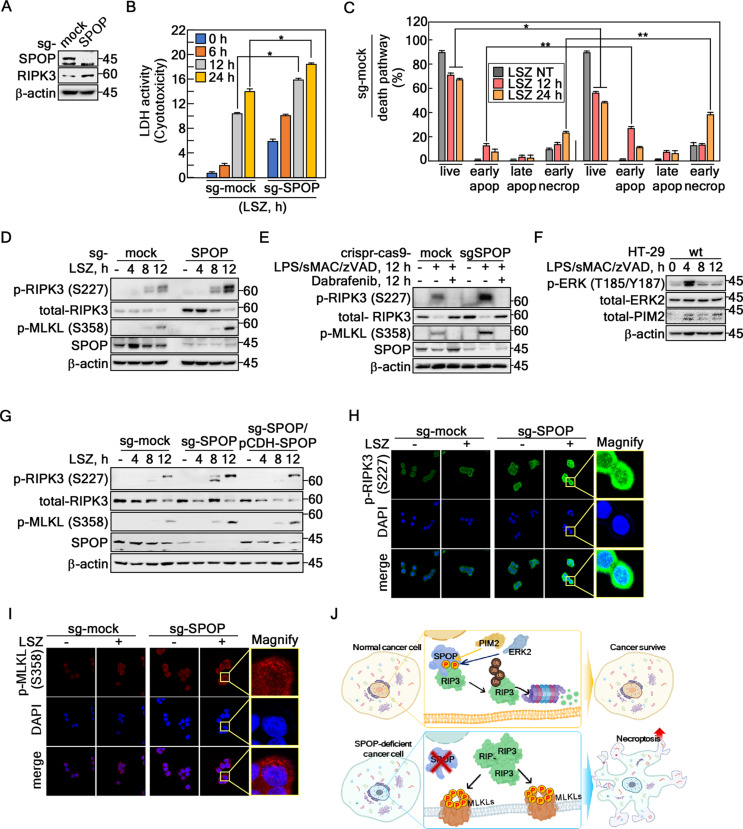
Fig. 7.
SPOP knockout sensitizes RIPK3-mediated necroptosis. A Confirmation of increased RIPK3 protein levels by SPOP knockout using small guide RNA-SPOP (sg-SPOP) in HT-29 cells. B Confirmation of the increased LSZ-induced cell toxicity of SPOP knockout cells by LDH activity analysis in HT-29 cells. C Death pathway tracking of LSZ-treated SPOP knockout cells using Annexin V and propidium Iodide staining by flow cytometry in HT-29 cells. D RIPK3 and MLKL phosphorylation was increased by the LSZ treatment in SPOP knockout HT-29 cells. E The activation of SPOP-RIPK3-mediated necroptosis signal molecules was abrogated by the Debrafenib (10 µM, 12 h) treatment. F Confirmation of the change in protein level of phospho-ERKs and total-PIM2 under the LSZ treatment at the indicated times. G Increased phospho-RIPK3 and -MLKL protein levels by LSZ treatment in sg-SPOP HT-29 cells abrogated by SPOP re-introduction into sg-SPOP HT-29 cells. H and I Illustrations showing that SPOP knockout increased LSZ-induced phospho-RIPK3 at Ser227 (H, ×400) and phospho-MLKL at Ser358 (I, ×400) by ICF under a confocal microscope in HT-29 cells. J The graphic abstract for this research indicates that SPOP acts as an E3 ligase for RIPK3, inhibiting necroptotic cell death in normal cancer cells. On the other hand, when the SPOP activity was depleted, increased RIPK3 protein accelerated necroptotic cell death when the cancer cells were stimulated with necroptosis inducers. A and D-G β-actin was used as an internal control for an equal protein loading. B and C The error bars from a triplicate experiment indicate SEM. * p < 0.05; ** p < 0.01 (Student t-test)