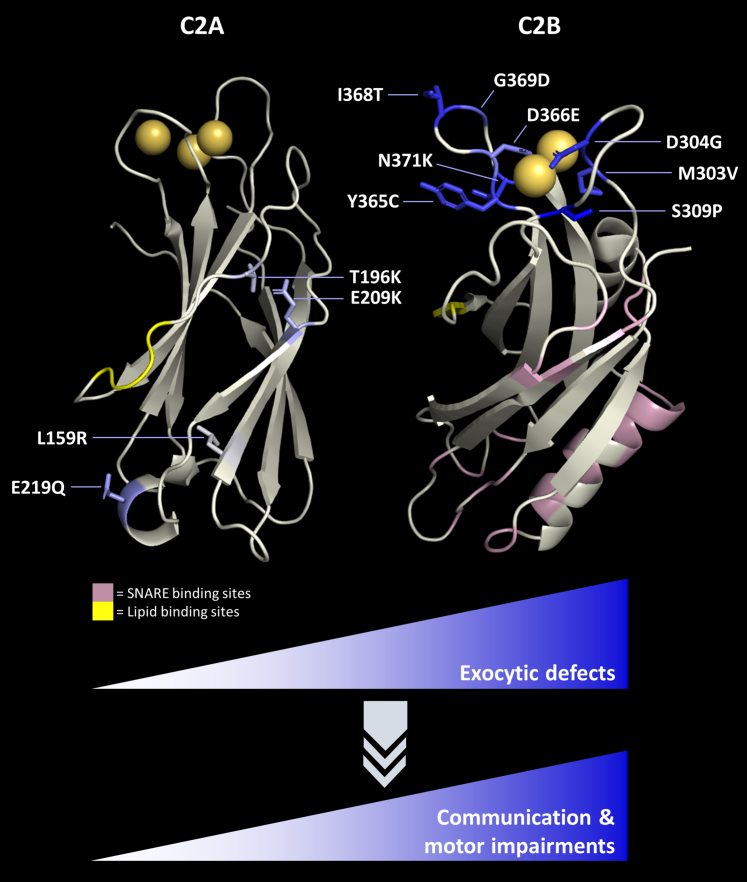
Fig. 7.
Genotype-function-phenotype relationship in SYT1-associated neurodevelopmental disorder. SYT1 variants in both the C2A domain (top left) and C2B domain (top right) cause graded, dominant-negative impairments to evoked exocytosis (this paper,29 colour of each residue equates to relative impact to exocytosis, gold spheres are calcium ions). The degree of impaired exocytosis correlates with motor and communication difficulties in individuals harbouring these variants, and is dependent on the nature of the amino acid substitution and its location within SYT1. SYT1 variants that perturb protein stability tend to present with stronger adaptive functions and fewer neurological symptoms, which may be due to either reduced expression or mitigation of dominant-negative effects. Known regions of functional importance are also indicated: polybasic lysine patches which bind to acidic phospholipids (yellow),20 and residues of the primary and tripartite interfaces between SYT1 and its SNARE protein binding partners (pink).23,50