Abstract
Yellow- and white-fleshed peach fruits are favored for their diverse flesh colors. While carotenoid accumulation primarily dictates flesh color differences, the influence of volatile compounds on their aromas remains largely unexplored. Here, multiple analytical methods including odor importance assessment, hierarchical clustering, and aroma characterization analysis were employed to investigate volatile compositions and aroma characteristics of the two types of peach, as well as the offspring with identical parentage. Dihydro-β-ionone was the sole volatile exhibiting content and odor importance disparities between the two types of peach, and in descendant cultivars such volatiles encompassed theaspirane additionally. Respectively 16 and 30 important volatiles were identified in the two peach types and in the offspring cultivars, and subsequently overview of their aroma characteristics was obtained from a graphical perspective. The two peach types and offspring cultivars all revealed prevalent floral, fruity and caramel notes, whereas the higher odor activity values and especially the woody odors in the white-fleshed cultivars, as well as the differential balance degrees of the main odor directions defined the distinct aromas. By delving into the pivotal differences in odor directions and aroma profiles between the two types of peach, this research elucidates the aroma distinctions rooted in flesh color variations and paves the way for uncovering aroma formation mechanisms in fruits with varied flesh colors.
Keywords: Peach, Offspring, Aroma, Flesh colors, Dihydro-β-ionone, Odor directions
Graphical abstract
Highlights
-
•
Only dihydro-β-ionone exhibited concentration and odor importance disparities between the two types of peach.
-
•
Respectively 16 and 30 volatiles were important to aromas of the two types of peach and the offspring cultivars.
-
•
Graphical overview of the aroma characteristics of the two types of peach and offspring cultivars was obtained.
-
•
The two types of peach and offspring cultivars exhibited the same prevalent odor notes.
-
•
Distinct aromas between the two peach types arose from the higher OAVs and woody odors in the white-fleshed type.
1. Introduction
Peach (Prunus persica L.) fruit are consumed worldwide for its attractive appearance and flavor quality, and yellow- and white-fleshed peach fruit are two major peach types significant and integral to the peach industry and appeal to consumers. Yellow- and white-fleshed peach cultivars are abundant and of high nutritious, economic and research values (Zhao et al., 2022), and yellow-fleshed peach fruit are suitable for both fresh and canned foods. These two peach types offer diversity in visual and gustatory perception of fruits, catering to a wider consumer base and contributing to market demand and season extension. Understanding the aroma differences between white- and yellow-fleshed peach is crucial for consumers seeking specific flavor profiles and for growers aiming to meet diverse market needs.
It is carotenoid accumulation that determines the color differences between white- and yellow-fleshed peach, the content of which was much higher in yellow-fleshed peach. Carotenoid meanwhile act as an important factor in determining aroma of fruit. Previous research has shown that carotenoid pigmentation patterns have profound effects on the terpene volatile compositions in peach or other fruits. Lower carotenoid levels and higher levels of norisoprenoid volatiles were observed in white-fleshed mutant ‘Redhaven Bianca’ peach fruit compared to the yellow-fleshed ‘Redhaven’ peach (Brandi et al., 2011). Apocarotenoid volatiles (norisoprenoids) that derived from carotenoids proportionally were altered relative to their precursors in near-isogenic carotenoid biosynthetic mutants of the parent Ailsa Craig (Lewinsohn et al., 2005a, Lewinsohn et al., 2005b; Vogel et al., 2010). Compared to the pale green-flesh Feicui pummelo, decreased carotenoid production was accompanied by increased accumulation of volatile terpenoids in flavedo of red-flesh Chuhong pummelo (Liu et al., 2015).
Beyond terpenes volatiles, scant research addressed whether other volatiles and aroma characteristics were affected in fruits with varying flesh colors, particularly in peach fruit. Robertson et al. (1990) reported the amounts of hexanal, trans-2-hexenal, linalooly, γ-decalactone and δ-decalactone were significantly higher in the selected white-fleshed peach cultivars than in yellow-fleshed peach. It was observed that fruity note lactones volatiles were of higher contents in red-fleshed peach cultivars, and grassy note C6 compounds showed greater accumulations in white-fleshed peach fruit (Xin et al., 2018). In our prior study, we examined the aroma distinctions between the white-fleshed peach cultivar ‘Hu Jing Mi Lu’ and the yellow-fleshed peach cultivar ‘Jin Yuan’, aiming to elucidate initial insights into aroma variations between two types of peach fruit (Liu et al., 2022). However, constrained by cultivar diversity and methodological singularities, our understanding of crucial volatiles and aroma characteristics between the two types of peach remains nebulous. A vague comprehension persists regarding the contributions of volatiles to the distinct flavors of white- and yellow-fleshed peach cultivars, as well as the significance of volatile compounds in these fruits.
The presence of hundreds of volatile compounds in fruit and the precise mechanisms underlying their biosynthesis both add to the complexity of the heritance of aromas. The study of the possible likeness between accessions and their offspring, as far as the flavor impact was concerned in tangerine, strawberry and etc. Fruits (Miyazaki et al., 2011; Rey-Serra et al., 2022). The ester methyl anthranilate which was present only in the mother cultivar ‘Mieze Schindler,’ was detectable in one fourth of the F1 population and the low degree of inheritance shows that this important compound is easily lost in the strawberry breeding process (Olbricht et al., 2008). Though the parental strawberry lines ‘232’ and ‘1392’ displayed similar relative content for alcohols and esters, and line ‘1392’ displayed higher contents of aldehydes, ketones, furans, and terpenes, the segregating progeny displayed even higher variation for most of the volatiles (Zorrilla-Fontanesi et al., 2012). In kiwifruit, the content of terpene 1,8-cineole was high in the female parent, and segregated in mapping population from about 50 ng/g to over 400 ng/g in fruit (Zeng et al., 2020). Aroma trait as a whole shows variable and low heritability, indicating the necessity to explore the inheritance patterns and inter-varietal relationships of specific volatile compounds.
To enhance our comprehension of aroma disparities between white- and yellow-fleshed peach fruit, this study utilized six representative yellow-fleshed and six white-fleshed cultivars. The selected yellow-fleshed peach cultivars are of certain genetic diversity, with ‘Babygold 7’ developed in the Americas, and ‘Cheng Xiang,’ ‘Jin Yuan,’ ‘Jin Xiu,’ ‘Ju Huang,’ and ‘Kanto 14’ developed in Asia. The selected white-fleshed peach cultivars include the representative material of Chinese peaches ‘Long Hua Shui Mi,’ the late-ripening cultivar ‘Xia Hui 8,’ the nationwide traditional famous peach cultivars ‘Bai Hua Shui Mi’ and ‘Shen Zhou Mi Tao,’ and etc. Among these cultivars, offspring cultivars with the same parent ‘Bai Hua Shui Mi’ were selected for separate aroma analysis, aiming to explore the aroma profiles of genetically related cultivars with varying flesh colors. Employment of multiple approaches including volatiles distribution analysis, odor importance evaluation, hierarchical clustering analysis and aroma characteristics analysis led to the identification of important volatiles in the two types of peach and the offspring cultivars, and the overview of their differentiated aroma characteristics from a graphical perspective was obtained. By meticulously examining volatile compounds and aroma characteristics across various cultivars, this study illuminates the subtle nuances of aroma intrinsic to peaches of diverse flesh colors. It not only deepens our comprehension of peach fruit attributes but also facilitates exploration into the intricate interplay between genetic background, flesh pigmentation, and aroma composition.
2. Materials and methods
2.1. Plant material and sampling
All peach cultivars (Prunus persica. L.) used in this study except ‘Shen Zhou Mi Tao’ were cultivated in the National Peach Germplasm Repository in Nanjing, Jiangsu province, China. ‘Shen Zhou Mi Tao’ (P. persica. L.) were cultivated in Shenzhou, Hebei province, China. There is a genetic relationship between cultivars used in this study: ‘Bai Hua Shui Mi’ is the direct hybrid maternal parent of yellow-fleshed cultivar ‘Jin Xiu’ and the indirect hybrid maternal parent of yellow-fleshed cultivar ‘Jin Yuan’; Meanwhile, ‘Bai Hua Shui Mi’ is the direct hybrid maternal parent of white-fleshed cultivar ‘Yin Hua Lu’ and the indirect hybrid maternal of white-fleshed cultivar ‘Xia Hui 8.’
Fruits of each cultivar were harvested at the ripe stage (Fig. 1) and transported carefully to the laboratory. For each cultivar eighteen fruits of uniform size and free of visible defects were selected, with six fruits in each biological replicate. The mesocarp was rapidly cut into pieces, frozen immediately in liquid nitrogen, and stored at −80 °C until assayed.
Fig. 1.
Picture of yellow- and white-fleshed peach fruit in this study. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
2.2. Detection of volatile compounds
Volatile compounds were detected according to the method described in Zhang et al. (2022). Samples were ground into a powder under liquid nitrogen, and 5 g (fresh weight) of each sample was weighed in the vial and placed in a water bath at 30 °C for 2 min, after which 4 mL of saturated NaCl solution and 10 μL of 2-octanol (0.0819 μg μL−1) as the internal standard were added into the vial and the mixture was homogenized. Vials were preincubated at 40 °C for 30 min under continuous agitation (500 rpm); then, volatiles were extracted for 30 min at the same temperature and agitation speed, through headspace solid-phase microextraction with a 65-μm polydimethylsiloxane-divinylbenzene fiber. After that, the fiber was desorbed in the GC injection port for 5 min in the splitless mode. The 7890 A GC chromatograph was equipped with a DB-wax column (30 m × 0.32 mm × 0.25 μm; J&W Scientific), with helium as the carrier gas at a constant flow of 1.6 mL min−1. The GC device was programmed at an initial temperature of 34 °C for 2 min, with a ramp of 2 °C min−1 up to 60 °C, then with a ramp of 5 °C min−1 up to 220 °C, and held for 2 min. The injection port, interface, and MS source temperatures were 250, 260, and 230 °C, respectively. The ionization potential was set at 70 eV, recorded by a 5975C mass spectrometer (Agilent Technologies, J&W Scientific, USA), and the scanning speed was 7 scans/sec.
Volatile compounds were identified by comparing the electron ionization mass spectra with the data from the NIST/EPA/NIH mass spectral library (NIST-08), and retention time data was also taken into consideration during the identification process. Lactone volatiles were also compared with injected standards (Sigma, USA). Concentrations of volatiles were determined using the peak area of the internal standard as a reference based on the total ion chromatogram.
2.3. Calculation of odor activity value
The odor activity value of each volatile compound was calculated as the ratio of its concentrations to its odor threshold, and the threshold was obtained from the literatures (Burdock, 2010; Zhang et al., 2022). The odor activity value was used to determine which volatiles were important for fruit odor (odor activity value > 1).
2.4. Analysis of the aroma characteristics of peach cultivars
The overall aroma characteristics of peach cultivars were analyzed based on the odor activity values of the identified important volatile compounds (Zhang et al., 2022). The odor notes of each important volatile compound were obtained and OAV summation values were calculated, after which the aroma characteristics were shown by radar map. Radar maps were prepared using Origin8.0 (Microcal Software).
2.5. Statistics
Hierarchical cluster analysis (HCA) was conducted using the online tool MetaboAnalyst 6.0 (http://www.metaboanalyst.ca/). Standard errors (SE) were calculated using Microsoft Excel 2019(Microsoft Corporation, Redmond, WA, USA). IBM SPSS Statistics 25 (IBM Corporation, Chicago, IL, USA) was used to conduct one-way ANOVA followed by Duncan's multiple range test at the 5% level (p < 0.05). Figures were prepared with Origin8.0 (Microcal Software).
3. Results
3.1. Distribution of volatile compounds in yellow- and white-fleshed peach cultivars derived from four aroma biosynthetic pathways
Volatile compounds of peach cultivars with different flesh colors were investigated in this study. Six yellow-fleshed peach cultivars ‘Cheng Xiang,’ ‘Jin Yuan,’ ‘Jin Xiu,’ ‘Ju Huang,’ ‘Kanto 14,’ and ‘Babygold 7,’ and six white-fleshed peach cultivars ‘Bai Hua Shui Mi,’ ‘Chun Xia Mi,’ ‘Yin Hua Lu,’ ‘Xia Hui 8,’ ‘Shen Zhou Mi Tao,’ and ‘Long Hua Shui Mi’ were used (Fig. 1). Volatile compounds detected in these peach cultivars were grouped depending on the volatile compound biosynthetic pathways (Schwab et al., 2008; Wang et al., 2014), including volatile fatty acid derivatives from the fatty acid pathways, terpenoid derivatives from terpenoid pathway, amino-acid volatile derivatives from shikimate and phenylpropanoid pathways, and lactones from β-oxidation pathways.
As shown in Table S1, a total 73 volatile compounds had the same distribution pattern in the cultivars of either the yellow- or white-fleshed peach type. Apart from these 73 volatile compounds, 40 volatile fatty acid derivatives, nine terpenoid derivatives, six amino-acid volatile derivatives and seven lactones were not uniformly distributed in either type, such as 1-octen-3-one, β-pinene, eugenol, δ-dodecalactone and etc.
Distributions of volatile compounds in the two types of peach cultivars were analyzed (Fig. S1). Respectively 20, four, six and two volatile compounds from the four groups coexisted in the white- and yellow-fleshed peach fruit, and no volatile compounds were observed to exist in single one type. In the group of volatile fatty acid derivatives, three volatiles including (Z)-3-hexenal, (Z)-2-hexenyl acetate, and dodecanoic acid were present, whereas another seven volatiles were absent in all six white-fleshed cultivars, and these ten volatiles were not present in all cultivars of the yellow-fleshed type cultivars. Meanwhile, four volatiles including nonanal, pentyl acetate, prenyl acetate and (E)-2-octen-1-ol were detected in all six yellow-fleshed cultivars, and another four volatiles were absent in the cultivars of this peach type, but the distribution patterns of above eight volatiles were different in the cultivars of the white-fleshed type of peach. Similarly, detailed distribution of volatile compounds in the group of terpenoid derivatives was also analyzed. For example, limonene was consistently present, and another nine volatiles were absent in all yellow-fleshed cultivars, and these ten volatiles were not consistently detected in the white-fleshed type cultivars. In the group of amino-acid volatile derivatives, α-n-hexyl cinnamic aldehyde was absent in the white-fleshed type cultivars, and was not consistently present in the cultivars of the yellow-fleshed type. Cinnamaldehyde was present, and another two volatiles were absent in the yellow-fleshed type fruit, and these three volatiles were not consistently detected in the white-fleshed type cultivars. In the group of lactones, δ-decalactone was consistently detected, and α-angelica lactone was absent in the white-fleshed type cultivars, and these two lactones were not consistently detected in the yellow-fleshed type cultivars. γ-Octalactone was consistently detected, and δ-nonalactone was absent in the yellow-fleshed type cultivars, and these two lactones were not consistently detected in the other type of fruit.
Over one-fifth of all detected volatile compounds from the four groups coexisted in both the yellow- and white-fleshed peach cultivars, and no volatiles were found to be existed in single one type of peach, suggesting that these two types of peach fruit may share more similar aroma characteristics, which was to be further investigated. The consistently detected volatiles in each type of cultivars (Table S1), including three volatile fatty acid derivatives in the white-fleshed type fruit and four volatile fatty acid derivatives in the yellow-fleshed type fruit, two terpenoid derivatives in the white-fleshed cultivars and one terpenoid derivative in the yellow-fleshed cultivars, one amino-acid volatile derivative in the yellow-fleshed cultivars, and one lactone in each type may contribute to the aroma differences between two types of peach. The rest detected volatiles were not uniformly distributed in the cultivars in each type, and they may give specific odor notes to the corresponding cultivars.
In the yellow-fleshed cultivars, ‘kanto 14’ and ‘Babygold 7’ are non-melting texture while the rest four cultivars are melting. By comparing the volatile compounds composition between these two different texture cultivars (Table S1), it was observed although the consistently detected volatiles varied in non-melting versus melting cultivars, no volatiles were exclusively present in either texture cultivars, and these cultivars share nearly forty volatiles regardless of textures, indicating the limited difference in volatiles composition between non-melting and melting yellow-fleshed peach fruit.
3.2. Analysis of volatile compounds in yellow- and white-fleshed descendant cultivars of ‘Bai Hua Shui Mi’
The two yellow-fleshed cultivars ‘Jin Xiu’ and ‘Jin Yuan,’ and two white-fleshed cultivars ‘Yin Hua Lu’ and ‘Xia Hui 8’ are genetically related and serve as good materials to investigate aroma differences between white- and yellow-fleshed peach cultivars from the same parent. As it shown, 28 volatile fatty acid derivatives, nine terpenoid derivatives, nine amino-acid volatile derivatives, three lactones coexisted in both the yellow- and white-fleshed descendant cultivars (Table S2), suggesting that the biosynthesis of these volatiles in peach fruit were under very limited impact of the difference in the formation mechanism between white and yellow flesh colors. Besides, volatiles from three groups were observed to be existed in single yellow- or white-fleshed offspring, including five volatile fatty acid derivatives (dodecanoic acid, n-hexadecanoic acid, (Z)-3-nonen-1-ol, tridecanoic acid, (E)-2-nonen-1-ol) exclusively present in the white-fleshed offspring and three volatile fatty acid derivatives (1-undecanol, 17-octadecynoic acid, (Z)-2-penten-1-ol) in the yellow-fleshed offspring, four terpenoid derivatives (dihydro-β-ionone, theaspirane, 6-methyl-5-hepten-2-ol, citral) in the white-fleshed offspring and one lactone (γ-octalactone) in the yellow-fleshed offspring. The occurrence of these exclusively present volatiles in single yellow- or white-fleshed offspring may be related with the biosynthetic mechanism of white and yellow flesh colors.
As shown in Fig. S2, the volatile compositions of the offspring cultivars were characterized by exclusively existed and more coexisted volatile compounds compared to that of the yellow- and white-fleshed cultivars. The increased coexisted volatile compounds included eight volatile fatty acid derivatives from Group 1, five terpenoid derivatives from Group 2, three amino-acid volatile derivatives from Group 3 and one lactone from Group 4.
3.3. Concentration differences of all detected volatiles between yellow- and white-fleshed peach cultivars as well as the offspring cultivars
Concentrations of all detected volatile compounds among the yellow- and white-fleshed peach cultivars were analyzed, and only one volatile compound dihydro-β-ionone from the group of terpenoid derivatives showed significant differences between the two types of fruit (Fig. 2A). Dihydro-β-ionone, a terpene volatile compound that was consistently present in the white-fleshed type cultivars (Table S1), was of higher concentrations in this type of fruit. For the rest more than 100 detected volatile compounds, the concentrations between these two types of cultivars showed no differences.
Fig. 2.
Volatile compounds with significant differences in concentrations between A, yellow- and white-fleshed peach cultivars, B, yellow- and white-fleshed offspring cultivars. Concentrations were calculated with an internal standard as the reference. CX, Cheng Xiang; JY, Jin Yuan; JX, Jin Xiu; JH, Ju Huang; K14, Kanto 14; B7, Babygold 7; BHSM, Bai Hua Shui Mi; CXM, Chun Xia Mi; YHL, Yin Hua Lu; XH8, Xia Hui 8; SZMT, Shen Zhou Mi Tao; LHSM, Long Hua Shui Mi. ‘Jin Yuan’ and ‘Jin Xiu’: yellow-fleshed offspring of ‘Bai Hua Shui Mi; ’ ‘Yin Hua Lu’ and ‘Xia Hui 8’: white-fleshed offspring of ‘Bai Hua Shui Mi.’ ND, not detected. FW, Fresh weight. SE values were calculated from three replicates, and comparisons were analyzed using one-way ANOVA followed by Duncan's multiple range test at the 5% level. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Furthermore, concentrations of all detected volatile compounds among the yellow- and white-fleshed offspring cultivars were also compared. Not only dihydro-β-ionone, but also 2-ethyl-1-hexanol, dodecanoic acid and (Z)-3-nonen-1-ol from the group of volatile fatty acid derivatives, and theaspirane and 6-methyl-5-hepten-2-ol from the group of terpenoid derivatives showed significant differences between the yellow- and white-fleshed offspring cultivars (Fig. 2B). 2-Ethyl-1-hexanol was present in all offspring cultivars (Table S2), and it was the only compound that showed significantly higher concentrations in the yellow-fleshed offspring cultivars. The other two volatile fatty acid derivatives and three terpenoid derivatives were all existed in single white-fleshed offspring cultivars (Table S2) and they were of significantly higher concentrations (Fig. 2B).
3.4. Odor importance evaluations of volatile compounds in yellow- and white-fleshed peach cultivars
The odor activity values of all detected volatile compounds among the yellow- and white-fleshed cultivars were calculated to obtain the odor important volatile compounds and 34 odor important volatiles were identified (Table 1). In Group 1–4, there were respectively 22, 7, 3 and 2 odor important volatiles.
Table 1.
Volatile compounds with odor activity values > 1 in yellow- and white-fleshed peach cultivars.
| Groups of Volatiles | Volatile compounds | Odor Threshold in |
Yellow-fleshed cultivars-OAV |
White-fleshed cultivars-OAV |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Water (μg/kg) | CX | JY | JX | JH | K14 | B7 | BHSM | CXM | YHL | XH8 | SZMT | LHSM | ||
| Volatile fatty acid derivatives |
Octanal | 1.4 | 2.0 | 3.7 | 5.0 | 4.7 | 6.5 | 1.6 | 2.0 | 1.0 | 3.0 | 6.0 | 1.6 | 2.4 |
| Decanal | 2 | 3.1 | 2.0 | 1.3 | 4.9 | 4.5 | 4.0 | 2.1 | 1.9 | 4.0 | 2.9 | 2.1 | 2.2 | |
| 2-Hexenal, (E)- | 30 | 15.4 | 22.4 | 41.1 | 13.1 | 15.3 | 5.8 | 11.8 | 27.4 | 22.1 | 39.7 | 30.7 | 45.1 | |
| 2-Octenal, (E)- | 3 | 2.7 | 5.0 | 6.2 | 8.0 | 7.5 | 3.6 | 4.3 | 1.3 | 6.7 | 7.5 | 5.2 | 3.0 | |
| 2-Nonenal, (E)- | 0.1 | 54.6 | 80.2 | 108.3 | 192.5 | 170.4 | 267.8 | 275.6 | 22.9 | 423.4 | 232.6 | 85.7 | 173.7 | |
| 2,6-Nonadienal, (E,E)- | 0.09 | 29.0 | 22.3 | 39.9 | 41.5 | 49.0 | 74.1 | 42.0 | 35.2 | 85.9 | 53.2 | 57.0 | 19.9 | |
| Hexyl acetate | 2 | 72.4 | 9.3 | 5.9 | 5.4 | 26.8 | 5.4 | 10.4 | 3.6 | 11.4 | 10.5 | 25.2 | 33.9 | |
| 3-Hexen-1-ol, acetate, (Z)- | 7.8 | 67.0 | 40.9 | 7.1 | 5.8 | 52.1 | 5.4 | 4.3 | 8.7 | 52.3 | 17.2 | 10.4 | 31.7 | |
| Heptanal | 3 | 1.6 | 2.1 | 2.9 | 3.5 | 4.3 | 3.6 | 2.5 | – | 2.9 | 3.0 | 2.2 | 1.7 | |
| Nonanal | 1 | 9.6 | 10.4 | 8.7 | 19.8 | 13.0 | 15.5 | 9.9 | 12.5 | 11.4 | 12.0 | 7.6 | – | |
| 3-Hexenal | 0.25 | – | 24.3 | 39.9 | 47.1 | 57.6 | 11.6 | 6.8 | 31.8 | 14.9 | 73.9 | 14.1 | 33.6 | |
| Hexanal | 4 | – | – | – | 53.9 | 56.3 | 34.0 | – | 79.6 | – | – | – | 317.3 | |
| 3-Octanone | 21 | – | – | – | – | – | – | 1.1 | – | 1.3 | 1.5 | – | – | |
| 3-Hexen-1-ol, (Z)- | 70 | – | – | – | – | – | – | – | – | – | – | – | 1.0 | |
| 2-Heptenal | 13 | 1.2 | 1.9 | 2.5 | 1.1 | 1.3 | – | 1.5 | – | 2.6 | 2.5 | 2.1 | – | |
| 2,4-Nonadienal, (E,E)- | 0.05 | 68.7 | 70.5 | 96.7 | – | 15.7 | 46.5 | 82.7 | – | 99.3 | 94.0 | 95.2 | 17.1 | |
| 1-Heptanol | 3 | – | – | 1.1 | – | 2.1 | 1.3 | 1.5 | – | – | 1.3 | – | – | |
| 1-Octanol, 2-butyl- | 0.00001 | 45,959.3 | – | 45,094.6 | – | – | – | 72,106.9 | 16,794.2 | – | – | 51,852.6 | – | |
| 2,4-Decadienal, (E,E)- | 0.07 | 4.3 | 26.3 | 25.1 | – | – | – | 18.5 | – | 37.1 | 27.4 | 33.0 | – | |
| 3,5-Octadien-2-one | 0.15 | 10.5 | 13.6 | – | – | 4.5 | – | – | – | – | – | 40.8 | – | |
| 1-Octen-3-one | 0.05 | – | 130.2 | – | 483.2 | 341.0 | 66.2 | 66.8 | – | 92.9 | 112.2 | – | – | |
| 1-Octen-3-ol |
14 |
– |
– |
– |
– |
1.3 |
1.5 |
2.5 |
– |
2.4 |
– |
1.2 |
– |
|
| Terpenoid derivatives |
trans-β-Ionone | 0.007 | 76.2 | 95.5 | 135.2 | 76.0 | 95.2 | 40.2 | 1041.0 | 202.0 | 111.6 | 1451.6 | 452.0 | 171.4 |
| Dihydro-β-ionone | 3.6 | – | – | – | – | – | – | 7.8 | 2.6 | 3.2 | 2.7 | 5.4 | 3.1 | |
| Theaspirane | 0.2 | – | – | – | – | – | – | 47.9 | – | 24.7 | 45.6 | – | – | |
| 2-Bornanone | 1 | – | 1.1 | 2.0 | – | – | – | – | – | – | 1.1 | 1.4 | – | |
| Linalool | 6 | 13.8 | 69.8 | 1.5 | – | – | 7.0 | 1.5 | – | 16.0 | 7.7 | – | 8.5 | |
| 6-Methyl-5-heptene-2-one | 50 | – | – | – | 1.1 | – | 1.1 | – | – | – | – | – | – | |
|
β-Damascenone |
0.002 |
571.7 |
138.1 |
202.6 |
– |
– |
– |
328.6 |
– |
502.3 |
328.0 |
– |
– |
|
| Amino-acid volatile derivatives |
Styrene | 3.6 | 3.3 | 1.1 | 3.1 | 3.9 | 1.4 | 1.4 | 1.6 | 1.8 | 2.0 | 1.8 | 1.5 | 1.7 |
| Benzaldehyde | 100 | – | 2.7 | 13.3 | 10.7 | – | 1.8 | 8.2 | 6.0 | 3.6 | 3.1 | 7.4 | 3.9 | |
| Benzyl Alcohol |
1.2 |
– |
– |
2.6 |
4.0 |
– |
2.3 |
2.5 |
– |
– |
2.2 |
2.0 |
– |
|
| Lactones | γ-Decalactone | 1 | 144.5 | 26.2 | 24.7 | 67.0 | 13.0 | 44.1 | 96.0 | 15.6 | 47.1 | 11.4 | 352.3 | 79.0 |
| γ-Octalactone | 7 | 1.1 | – | – | 2.0 | – | – | – | – | – | – | 2.8 | 0.2 | |
Note: OAV, odor activity value; -, odor activity value < 1. CX, Cheng Xiang; JY, Jin Yuan; JX, Jin Xiu; JH, Ju Huang; K14, Kanto 14; B7, Babygold 7; BHSM, Bai Hua Shui Mi; CXM, Chun Xia Mi; YHL, Yin Hua Lu; XH8, Xia Hui 8; SZMT, Shen Zhou Mi Tao; LHSM, Long Hua Shui Mi.
Dihydro-β-ionone, which was consistently present in the white-fleshed type cultivars (Table S1) and the only one volatile compound that showed significant differences in concentrations between the two types of fruit (Fig. 2A), was also odor important for the white-fleshed type peach fruit (Table S2).
In the group of volatile fatty acid derivatives, eight volatiles including octanal, decanal, (E)-2-hexenal, (E)-2-octenal, (E)-2-nonenal, (E,E)-2,6-nonadienal, hexyl acetate and (Z)-3-hexen-1-ol acetate showed odor importance in both the yellow- and white-fleshed peach cultivars, and another two volatiles including heptanal and nonanal showed consistent odor importance only to the yellow-fleshed peach cultivars, and another one volatile 3-hexenal showed consistent odor importance only to the white-fleshed peach cultivars (Table 1, Fig. S3). The rest volatile fatty acid derivatives didn't show consistent odor importance in either type of peach cultivars. In the group of terpenoid derivatives, trans-β-ionone was the only terpene that showed odor importance in both two types of peach cultivars. Dihydro-β-ionone showed odor importance only to the white-fleshed peach cultivars (Table 1). In the group of amino-acid volatile derivatives, styrene was the only volatile that showed odor importance in both two types of peach cultivars, and benzaldehyde showed consistent odor importance to the white-fleshed peach cultivars (Table 1). In the group of lactones, γ-decalactone showed odor importance both to the two types of peach cultivars (Table 1).
As Fig. S3 showed, the number of volatiles that showed odor importance in both the yellow- and white-fleshed peach cultivars was greater than that of the volatiles that showed consistent odor importance in one type of peach cultivars, indicating the common odors between two types of peach fruit. As the only volatile that showed odor importance to single one type of fruit, dihydro-β-ionone contributed to the odor differences between two types of fruit. Moreover, the different volatiles that showed consistent odor importance to one type of peach also gave varied odors to these two types of fruit.
The common top odor important volatiles in two types of fruit were determined based on odor activity values. Trans-β-Ionone, (E)-2-nonenal, γ-decalactone, (E,E)-2,6-nonadienal and (Z)-3-hexen-1-ol acetate were among the top five odor important volatiles in the white-fleshed peach cultivars (Table 1). And for the yellow-fleshed peach cultivars, trans-β-ionone, (E)-2-nonenal, γ-decalactone and (Z)-3-hexen-1-ol acetate still remained the top five odor important volatiles, and the only different top odor important volatile was hexyl acetate.
3.5. Odor importance evaluations of volatile compounds in yellow- and white-fleshed offspring cultivars
In the yellow- and white-fleshed offspring cultivars, the distribution of odor important volatiles was also analyzed and 30 odor important volatiles were identified (Table 2).
Table 2.
Volatile compounds with odor activity values > 1 in yellow- and white-fleshed offspring cultivars.
| Groups of Volatiles | Volatile compounds | Yellow-fleshed offspring-OAV |
White-fleshed offspring-OAV |
||
|---|---|---|---|---|---|
| JY | JX | YHL | XH8 | ||
| Volatile fatty acid derivatives |
Octanal | 3.7 | 5.0 | 3.0 | 6.0 |
| Decanal | 2.0 | 1.3 | 4.0 | 2.9 | |
| 2-Hexenal, (E)- | 22.4 | 41.1 | 22.1 | 39.7 | |
| 2-Octenal, (E)- | 5.0 | 6.2 | 6.7 | 7.5 | |
| 2-Nonenal, (E)- | 80.2 | 108.3 | 423.4 | 232.6 | |
| 2,6-Nonadienal, (E,E)- | 22.3 | 39.9 | 85.9 | 53.2 | |
| Hexyl acetate | 9.3 | 5.9 | 11.4 | 10.5 | |
| 3-Hexen-1-ol, acetate, (Z)- | 40.9 | 7.1 | 52.3 | 17.2 | |
| Heptanal | 2.1 | 2.9 | 2.9 | 3.0 | |
| Nonanal | 10.4 | 8.7 | 11.4 | 12.0 | |
| 3-Hexenal | 24.3 | 39.9 | 14.9 | 73.9 | |
| 2-Heptenal | 1.9 | 2.5 | 2.6 | 2.5 | |
| 2,4-Nonadienal, (E,E)- | 70.5 | 96.7 | 99.3 | 94.0 | |
| 2,4-Decadienal, (E,E)- | 26.3 | 25.1 | 37.1 | 27.4 | |
| 3-Octanone | – | – | 1.3 | 1.5 | |
| 1-Octen-3-one | 130.2 | – | 92.9 | 112.2 | |
| 1-Heptanol | – | 1.1 | – | 1.3 | |
| 1-Octanol, 2-butyl- | – | 45,094.6 | – | – | |
| 3,5-Octadien-2-one | 13.6 | – | – | – | |
| 1-Octen-3-ol |
– |
– |
2.4 |
– |
|
| Terpenoid derivatives |
trans-β-Ionone | 95.5 | 135.2 | 111.6 | 1451.6 |
| Linalool | 69.8 | 1.5 | 16.0 | 7.7 | |
| β-Damascenone | 138.1 | 202.6 | 502.3 | 328.0 | |
| Dihydro-β-ionone | – | – | 3.2 | 2.7 | |
| Theaspirane | – | – | 24.7 | 45.6 | |
| 2-Bornanone |
1.1 |
2.0 |
– |
1.1 |
|
| Amino-acid volatile derivatives |
Styrene | 1.1 | 3.1 | 2.0 | 1.8 |
| Benzaldehyde | 2.7 | 13.3 | 3.6 | 3.1 | |
| Benzyl Alcohol |
– |
2.6 |
– |
2.2 |
|
| Lactones | γ-Decalactone | 26.2 | 24.7 | 47.1 | 11.4 |
Note: OAV, odor activity value; -, odor activity value < 1. JY, Jin Yuan; JX, Jin Xiu; YHL, Yin Hua Lu; XH8, Xia Hui 8. ‘Jin Yuan’ and ‘Jin Xiu’: yellow-fleshed offspring of ‘Bai Hua Shui Mi’; ‘Yin Hua Lu’ and ‘Xia Hui 8’: white-fleshed offspring of ‘Bai Hua Shui Mi’.
Among the six volatile compounds that showed significant differences in concentrations between the yellow- and white-fleshed offspring cultivars (Fig. 2B), theaspirane and dihydro-β-ionone were odor important for the white-fleshed offspring cultivars, whereas the other four volatile compounds were not odor important in all offspring cultivars (Table 2).
Apart from the 11 volatiles that showed odor importance in two types of fruit (Fig. S3), additional six volatile fatty acid derivatives including heptanal, nonanal, 3-hexenal, 2-heptenal, (E,E)-2,4-nonadienal, and (E,E)-2,4-decadienal, and two terpenoid derivatives including linalool and β-damascenone, and one amino-acid volatile derivative benzaldehyde showed odor importance to all yellow- and white-fleshed offspring cultivars (Table 1, Table 2). Moreover, another one volatile fatty acid derivative 3-octanone and one terpene theaspirane showed odor importance to single one type of fruit, to endow the different odors in the white-fleshed offspring fruit.
Meanwhile, the common top odor important volatiles in the yellow- and white-fleshed offspring cultivars were compared. These offspring cultivars shared four top odor important volatiles including trans-β-ionone, β-damascenone, (E)-2-nonenal and (E,E)-2,4-nonadienal, though the OAVs in the white-fleshed offspring cultivars were higher. Besides, linalool and 1-octen-3-one were among the top odor important volatiles respectively in the yellow- and white-fleshed offspring cultivars.
3.6. Important volatiles identified in yellow- and white-fleshed as well as in the offspring cultivars
Volatile compounds with significant differences in concentrations among the yellow- and white-fleshed peach fruit as well as the offspring cultivars, and the odor important volatiles within these cultivars were identified (Fig. 2, Table 1, Table 2). These volatiles played an important role to exhibit the aroma characteristics to corresponding cultivars. For the yellow- and white-fleshed peach fruit, there were in total 16 volatiles that showed odor importance for at least one type of peach fruit and the only one volatile that showed significant differences in concentrations between two types of cultivars was also included. For the yellow- and white-fleshed offspring cultivars, significance analysis of volatile compounds concentrations between two types of peach and OAV value analysis led to the identification of 30 volatile compounds.
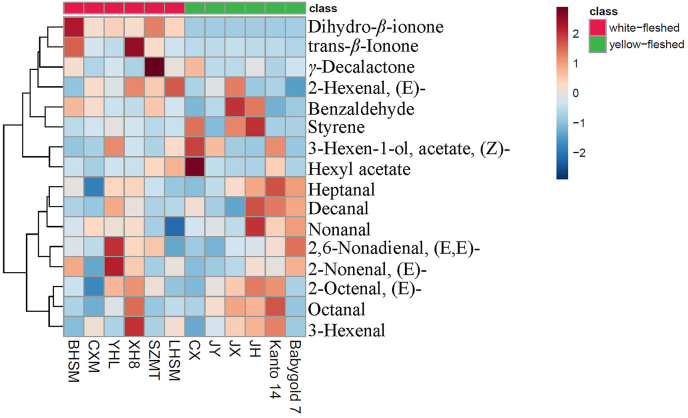
Concentrations of the identified important volatiles in yellow- and white-fleshed as well as in the offspring cultivars were subjected to Hierarchical cluster analysis to reveal the clustering and differences in volatiles profiles among cultivars, and heatmaps of the volatiles profiling were shown (Fig. 3, Fig. 4). The HCA heatmap clearly suggested the yellow-fleshed peach cultivars were characterized by the majority important volatile fatty acid derivatives which tended to be of higher concentrations in this type of peach, such as (Z)-3-hexen-1-ol acetate, hexyl acetate, heptanal, decanal, nonanal, etc. (Fig. 3). The white-fleshed peach cultivars were different from the yellow-fleshed cultivars due to the higher concentrations of two important terpenes (dihydro-β-ionone and trans-β-ionone) and one important lactone γ-decalactone (Fig. 3).
Fig. 3.
Heatmap analysis of concentrations of the identified 16 important volatile compounds in the yellow- and white-fleshed peach fruit. BHSM, Bai Hua Shui Mi; CXM, Chun Xia Mi; YHL, Yin Hua Lu; XH8, Xia Hui 8; SZMT, Shen Zhou Mi Tao; LHSM, Long Hua Shui Mi; CX, Cheng Xiang; JY, Jin Yuan; JX, Jin Xiu; JH, Ju Huang. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
Heatmap analysis of concentrations of the identified 30 important volatile compounds in the yellow- and white-fleshed offspring peach fruit. YHL, Yin Hua Lu; XH8, Xia Hui 8; JY, Jin Yuan; JX, Jin Xiu. ‘Yin Hua Lu’ and ‘Xia Hui 8’: white-fleshed offspring of ‘Bai Hua Shui Mi; ’ ‘Jin Yuan’ and ‘Jin Xiu’: yellow-fleshed offspring of ‘Bai Hua Shui Mi.’. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
A clear distinction in the distribution of the important volatile compounds in the yellow- and white-fleshed offspring cultivars was observed. The white-fleshed offspring cultivars tended to have higher concentrations of a cluster of volatiles such as nonanal, hexyl acetate, (E)-2-nonenal, dodecanoic acid, (E,E)-2,6-nonadienal, (Z)-3-nonen-1-ol and etc., from the group of volatile fatty acid derivatives, and β-damascenone, dihydro-β-ionone, theaspirane, 6-methyl-5-hepten-2-ol and etc., from the group of terpenoid derivatives (Fig. 4). In comparison, fewer volatile compounds in the yellow-fleshed offspring cultivars tended to have higher concentrations, such as 2-bornanone and 2-ethyl-1-hexanol (Fig. 4). Some important volatiles were not consistently distributed in either type of offspring cultivars, including γ-decalactone, (Z)-3-hexen-1-ol acetate, (E)-2-hexenal and etc.
3.7. Peach odor characteristics of the yellow- and white-fleshed cultivars as well as the offspring fruit
The overall aroma characteristics of yellow- and white-fleshed cultivars as well as the offspring fruit were analyzed based on the odor activity values of the identified important volatile compounds. Aromas of yellow- and white-fleshed peach cultivars were characterized by eight odors including herbaceous, chemical, caramel, fruity, nutty, floral, spicy and woody, and the OAV summation values in the white-fleshed cultivars were much higher than those in the yellow-fleshed cultivars (Fig. 5).
Fig. 5.
Radar map of the aroma characteristics of the yellow- and white-fleshed peach cultivars. Axis scales in the upper two rows (yellow-fleshed type maps) were from 0 to 420, and in the lower two rows (white-fleshed type maps) were from 0 to 1800. CX, Cheng Xiang; JY, Jin Yuan; JX, Jin Xiu; JH, Ju Huang; K14, Kanto 14; B7, Babygold 7; BHSM, Bai Hua Shui Mi; CXM, Chun Xia Mi; YHL, Yin Hua Lu; XH8, Xia Hui 8; SZMT, Shen Zhou Mi Tao; LHSM, Long Hua Shui Mi. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Odors of the highest OAV summation values in the yellow-fleshed peach cultivars were caramel, fruity, floral and chemical, and in each yellow-fleshed cultivar values of these four odors were relatively balanced. By contrast, odors of the highest OAV summation values in the white-fleshed peach cultivars were floral, fruity, woody and caramel, and among them the first three odors were highlighted with values exceeding 1000 in some cultivars such as ‘Bai Hua Shui Mi’ and ‘Xia Hui 8,’ and the last odor caramel was of comparatively similar values with those in the yellow-fleshed cultivars (Fig. 5).
From a graphical perspective, bounded by the chemical, caramel, fruity and floral directions, the radar maps of yellow-fleshed cultivars were characterized by almost symmetrical graphics in the first and fourth quadrants, as well as almost symmetrical lines in the third and fourth quadrants (Fig. 5). Differently, for the white-fleshed cultivars the radar maps were characterized by almost symmetrical lines in the floral, fruity and woody directions, and most of these three lines were the longest in these radar maps (Fig. 5). On the whole, the yellow- and white-fleshed cultivars shared the prominent floral, fruity and caramel odors, and what distinguished these two types of cultivars were the woody odors in the white-fleshed cultivars and moreover, lied in the degree of balance of the OAV summation values in the main odor directions.
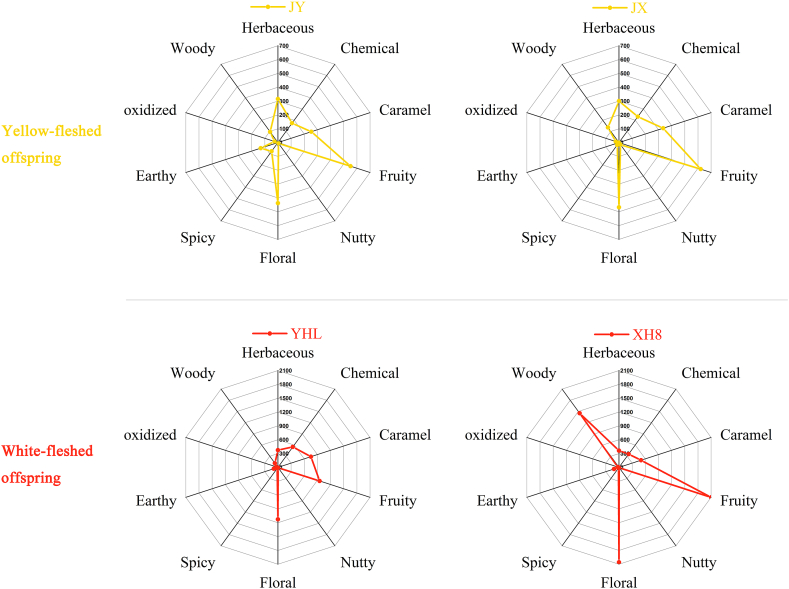
It was observed that aromas of the yellow- and white-fleshed peach offspring cultivars were characterized by two more odors which were earthy and oxidized odors apart from the below eight odors: herbaceous, chemical, caramel, fruity, nutty, floral, spicy and woody. Similarly, the OAV summation values in the white-fleshed offspring cultivars were much higher than those in the yellow-fleshed cultivars (Fig. 6). Despite the huge differences between the OAV summation values in two types of offspring cultivars, odors with the highest OAV summation values in each type were the same fruity, floral and caramel odors.
Fig. 6.
Radar map of the aroma characteristics of the yellow- and white-fleshed offspring cultivars. Axis scales in the upper rows (yellow-fleshed offspring maps) were from 0 to 700, and in the lower row (white-fleshed offspring maps) were from 0 to 2100. JY, Jin Yuan; JX, Jin Xiu; YHL, Yin Hua Lu; XH8, Xia Hui 8. (For interpretation of the references to color in this figure legend, the reader is referred to the Web version of this article.)
Among these offspring cultivars, aroma characteristics were highlighted by specific cultivars. For example, aroma of ‘Xia Hui 8’ was characterized by the very high values of woody odor, which was not uniformly distributed among the white-fleshed offspring cultivars (Fig. 6). Besides, the distributions of various odors in the white-fleshed offspring cultivars were much more uneven.
4. Discussion
4.1. Fruit terpenoid derivatives formation are related to different flesh colors
Previous research in fruit like tomato has indicated that some important fruit aroma volatiles were derived from the degradation of carotenoid pigments, suggesting the close association between carotenoid pigmentation and volatile terpene content (Lewinsohn et al., 2005a,b). 6-Methyl-5-hepten-2-one and β-ionone were only detected in red flesh papaya cultivar ‘Sui hong’ fruit, which exhibited significantly higher carotenoid contents and higher expression levels of carotenoid cleavage dioxygenase gene compared to the yellow flesh cultivar ‘Sui Huang’ (Jing et al., 2015). The carotenoids and aroma volatile apocarotenoids in three types of apricot cultivars (orange flesh-‘HY’ and ‘DX,’ yellow flesh-‘AK’ and ‘SL,’ light-yellow flesh-‘LT’ and ‘BX’) were determined, and the highest total carotenoid content was observed in the orange cultivars, while the total carotenoid contents detected in the yellow and light-yellow cultivars were only 27–63% and 19–20% respectively; meanwhile contents of β-ionone, dihydro-β-ionone, 6-methyl-hepten-2-one and β-damascenone remained very low level in orange cultivars, but higher in yellow flesh cultivars and the highest in the light-yellow flesh cultivars (Xi et al., 2020).
Correspondingly in this study, terpenoid derivatives was not only the sole group of volatile compounds that showed significantly different contents between yellow- and white-fleshed peach cultivars, but also the only group of volatile compounds that contained odor important volatile in single one-type of peach cultivars. Out of the more than 100 detected volatile compounds, dihydro-β-ionone was the only compound that showed significant differences between the two types of fruit (Fig. 2A) and was of higher concentrations in the white-fleshed type cultivars. Moreover, dihydro-β-ionone was the only volatile that showed odor importance to single one type of fruit (the white-fleshed cultivars) (Fig. S3). Above results drawn from this study were in accordance with the previous research concerning the differences of terpenes composition between fruits with different flesh colors, and this study underlined the importance of dihydro-β-ionone. Thus, more attention needs to be paid to the contribution of this volatile compound in future efforts to improve peach aroma.
Dihydro-β-ionone is naturally found in apricots, strawberries, blackberries, green beans and mushroom (Xu et al., 2019), constitutes the signatures of the fragrance of the rose species (Noh et al., 2024), and due to the mellow, sweet, and fresh cedar scent, it has received great attention from the flavor and fragrance industry and was widely used in foodstuff and beverage industries. As this study showed, though trans-β-ionone was the same common top odor important volatiles in two types of peach cultivars, its hydrogenation product dihydro-β-ionone was consistently present in the white-fleshed type cultivars (Table S1), and was only detected in partial yellow-fleshed cultivars with low contents. The content changes of β-ionone and dihydro-β-ionone in peach fruit were also focused by researchers. Liu et al. (2022) investigated the change of norisoprenoid volatiles under UV-B irradiation in the white-fleshed peach cultivar ‘Hujingmilu’, and found the content of dihydro-β-ionone and β-ionone increased significantly after 48 h of storage, but after 48 h of UV-B irradiation β-ionone content was nearly undetected and dihydro-β-ionone content decreased dramatically.
4.2. Obvious differences manifested in the composition and genetic trends of volatiles between parent-offspring cultivars
In this study, the comparative analysis of yellow- and white-fleshed peach cultivars with the same parent provides a perspective on the inheritance of aroma in parent-offspring generations and the aroma characteristics of specific cultivars. The odor important volatile compositions in the yellow-fleshed offspring cultivars of ‘Bai Hua Shui Mi’ tended to be different from those in the non-offspring yellow-fleshed cultivars, such as hexanal and γ-octalactone (Table 1). The similar distribution pattern was observed between the white-fleshed offspring cultivars and non-offspring white-fleshed cultivars, indicating the genetic tendency of certain specific volatiles in ‘Bai Hua Shui Mi’ towards offspring cultivars.
This study highlighted the differences of volatile compositions between yellow- and white-fleshed offspring cultivars (Fig. 2, Fig. 4, Fig. S3), though they have the same maternal parent. The importance of 2-ethyl-1-hexanol in the group of volatile fatty acid derivative was observed as it was the only volatile compound that showed higher concentrations in the yellow-fleshed offspring cultivars though detected in all offspring cultivars (Table S2, Fig. 2B). 2-Ethyl-1-hexanol tended to have higher contents in fruits at early developmental stages, such as in papaya fruit (Hadi et al., 2013) and pulp of umbu fruit (Galvão et al., 2011), and 2-ethyl-1-hexanol decreased significantly in concentrations with the advance of umbu fruit maturity. Besides, researchers also reported the importance of this compound in specific fruit cultivars. As observed in grape, 2-ethyl-1-hexanol was closely related to aroma of Carlos, the muscadine grape cultivar (Deng et al., 2021).
In the white-fleshed offspring cultivars, apart from the higher contents of dihydro-β-ionone which was consistent with the results observed in the white-fleshed cultivars (Fig. 2A), another two terpenoid derivatives theaspirane and 6-methyl-5-hepten-2-ol were also of significantly higher contents compared to those in the yellow-fleshed offspring cultivars (Fig. 2B). Theaspirane is described as having a pinewood- and menthol-like odor, and it was detected in raspberry, wild cherry, flat peaches (Li et al., 2023) and other fruits. By investigating the effects of 1-methylcyclopropene treatment on the storage quality and volatile compounds of Xiaobai apricot fruit stored at 4 °C, it was found that 1-MCP treatment could maintain the theaspirane content in apricot (Lv et al., 2021). 6-Methyl-5-hepten-2-ol was suspected to be lycopene degradation products, which was likely downstream products of 6-methyl-5-heptene-2-one, and it is abundant in red ripe tomato fruit; the correlation analysis of apocarotenoid in red fruits identified that 6-methyl-5-hepten-2-ol content was highly correlated with 6-methyl-5-heptene-2-one and trans-β-ionone contents (Gao et al., 2008; Zhang et al., 2024). It is worth noting that apart from the significantly higher contents of terpenoid derivatives in the white-fleshed offspring cultivars, dodecanoic acid and (Z)-3-nonen-1-ol from the group of volatile fatty acid derivatives also showed distinct contents (Fig. 2B). These two volatiles were all existed in single white-fleshed offspring cultivars (Table S2), just in the same distribution pattern with that of theaspirane and 6-methyl-5-hepten-2-ol, showing the potential interactions between the formation of flesh colors and the inheritance of volatiles derived from multiple biosynthetic pathways in peach.
By evaluating the odor important volatiles, volatile fatty acid derivative 3-octanone and two terpenoid derivatives dihydro-β-ionone and theaspirane were identified as odor important to merely the white-fleshed offspring cultivars (Table 2), showing their contributions to the different aromas of this type of offspring fruit. Moreover, the higher OAVs values of the common top important volatiles in the white-fleshed offspring cultivars also indicated the difference in aroma inheritance tendency between the offspring of two flesh-colored peaches.
4.3. Odor important volatiles identified in yellow- and white-fleshed cultivars: more similarities than differences
The number of odor important volatiles in both two types of peach cultivars in this study constituted the majority sum of all odor important volatiles detected, meanwhile few volatiles exhibited the limited differences between two types of cultivars, including dihydro-β-ionone and several volatiles that showed consistent odor importance to one type of cultivars. Dihydro-β-ionone was the main focus as this study revealed to show the aroma differences between two types of peach cultivars, and it has been discussed of its odor note and content changes in fruit above.
Odor important volatiles in both two types of cultivars covered all four groups of volatiles, which were aldehydes and esters in the group of volatile fatty acid derivatives, trans-β-ionone in the group of terpenoid derivatives, styrene in the group of amino-acid volatile derivatives, and γ-decalactone in the group of lactones. Meanwhile, trans-β-ionone, (E)-2-nonenal, γ-decalactone and (Z)-3-hexen-1-ol acetate were the common top odor important volatiles to both two types of cultivars, indicating their similar roles in the overall aroma formation in two types of cultivars. Based on the reported odor notes of above volatiles, it is obvious that these common odor important volatiles in both two types of peach cultivars impart the corresponding green, fruity, flower and peach-like aromas to both the yellow- and white-fleshed cultivars.
Lactones has been the focus of researchers due to their contributions to the peach-like odors in fruit, and as this study showed, γ-decalactone was the only lactone that contributed to the peach-like aromas of yellow- and white-fleshed cultivars indiscriminately, and the differences of its roles lay in the OAV values in specific cultivars, other than the type of peach cultivars with distinct flesh colors. This is consistent with our previous research findings concerning the odor important lactones volatile compounds in white-fleshed ‘Hu Jing Mi Lu’ and yellow-fleshed ‘Jin Yuan’ (Liu et al., 2022). Collectively, the contributions of volatile compounds to the differences in flavor between yellow- and white-fleshed peach cultivars may be more reflected in the content of specific volatiles, rather than the composition of unique volatile compounds. Therefore, peach germplasm with distinct flesh colors may also serve as ideal materials for in-depth research on the biosynthesis mechanism of volatile compounds.
4.4. Overview of aroma characteristics of yellow- and white-fleshed peach cultivars as well as offspring from a graphical perspective
The overall aroma characteristics of two types of peach cultivars as well as the offspring cultivars in this study were obtained based on the identified important volatiles in this study, and provided an approach to analyze and compare aroma differences through the intuitive visualization perspective, which has been widely used currently. Odors detected in the Four Nationwide Traditional Famous Peach cultivars were compared and radar maps of the aroma characteristics of each peach cultivar were present clearly (Zhang et al., 2022). Radar maps of the aroma characteristics of the fruit samples based on OAVs were applied to investigate the influence of 1-MCP treatment in flat peach samples, and it was shown that during the storage life of ‘Yulu’ flat peach fruit, 1-MCP markedly reduced the fruity and floral aromas (Zhang et al., 2023). Based on the e-nose data set and sensory evaluation of the four most popular types of red-cooked chicken, the different shapes of the radar map indicated significant differences in the volatilization intensity and proportion of various gases of red-cooked chicken samples and brands, and suggested that aroma of the four most popular redcooked chickens was determined by the balance and interaction among multiple aroma compounds (Sun et al., 2023).
As analyzed above, the shared predominant floral, fruity and caramel odors between yellow- and white-fleshed cultivars shaped the common basic aromas, while the higher OAVs values and especially the woody odors in the white-fleshed cultivars, as well as the differential balance degrees of the OAV summation values in the main odor directions defined the diversity of aromas between two types of fruit. Compared to the aroma differences, there were more similarities. And such result was in consistence with that reflected in kiwifruit: cultivars with different flesh colors cannot be effectively distinguished by their aroma chemical compositions, and different species of kiwifruit can be distinguished to some extent by their aroma chemical compositions, but the effect was not satisfactory (Lan et al., 2021), suggesting the aromas of kiwifruit with different flesh colors were not that distinctive.
This research also indicated that the offspring cultivars showed specific aroma characteristics. For example, ‘Xia Hui 8’ is a late-ripening peach cultivar bred by the team of the researcher in this study, and this cultivar is of the advantages of high yield, good color, firm texture, sweet flavor, slow softening and etc. (Yu et al., 2014). Aroma characteristics analysis showed ‘Xia Hui 8’ was of very high values of the woody odor among the offspring cultivars, and higher than that of the parent ‘Bai Hua Shui Mi.’
5. Conclusion
In summary, dihydro-β-ionone contributed to the aroma differences between the yellow- and white-fleshed peach cultivars, whereas between the two types of offspring cultivars they were dihydro-β-ionone and theaspirane. Totally 16 volatiles showed importance to aromas of the two types of peach cultivars, and to the two types of offspring cultivars there were 30 volatiles. The two types of peach cultivars as well as the offspring cultivars all exhibited prevalent floral, fruity, and caramel notes. Differences arose from the higher OAVs and particularly woody odors in the white-fleshed cultivars, and variations in the balance of primary odor directions between the two peach types. This research suggests the aroma diversity between the two peach types may be more evident in the profiles of specific volatile compounds rather than the composition of unique volatiles. By elucidating volatile compositions and aroma differentials between the two peach types, this research is crucial for future advancements in unraveling the mechanisms behind aroma formation in fruits, as well as in breeding and cultivation practices.
CRediT authorship contribution statement
Yuanyuan Zhang: Conceptualization, Data curation, Investigation, Methodology, Project administration, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing, Funding acquisition. Binbin Zhang: Conceptualization, Investigation, Resources, Validation, Visualization. Zhixiang Cai: Data curation, Software, Resources. Zhijun Shen: Conceptualization, Methodology, Resources, Funding acquisition. Mingliang Yu: Data curation, Investigation, Project administration, Resources, Validation, Writing – review & editing. Ruijuan Ma: Conceptualization, Investigation, Methodology, Project administration, Supervision, Resources, Validation, Writing – review & editing, Funding acquisition.
Declaration of competing interest
The authors declare that they have no conflict of interest.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (32002020; 32472714); the Earmarked Fund for China Agriculture Research System (CARS-30); and the Open Competition Project of Seed Industry Revitalization of Jiangsu Province (JBGS (2021)016). The authors sincerely thank Jiuqing Zhang at LiangSang Breeding Farm (Shenzhou, Hebei Province, China) for the kind help in the acquisition of fruit materials for the research, and undergraduate interns Jiarui Zhu and Wen Li for their assistance in the analysis of fruit volatile compounds.
Handling Editor: Dr. Maria Corradini
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.crfs.2024.100901.
Supplementary material
.
Appendix A. Supplementary data
The following are the Supplementary data to this article.
Data availability
Data will be made available on request.
References
- Brandi F., Bar E., Mourgues F., Horváth G., Turcsi E., Giuliano G., Liverani A., Tartarini S., Lewinsohn E., Rosati C. Study of ‘Redhaven’ peach and its white-fleshed mutant suggests a key role of CCD4 carotenoid dioxygenase in carotenoid and norisoprenoid volatile metabolism. BMC Plant Biol. 2011;11:24. doi: 10.1186/1471-2229-11-24. http://www.biomedcentral.com/1471-2229/11/24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdock G.A. sixth ed. CRC Press/Taylor & Francis; Boca Raton: 2010. Fenaroli's Handbook of Flavor Ingredients. [DOI] [Google Scholar]
- Deng H.H., He R.M., Long M.C., Li Y.M., Zheng Y.Y., Lin L.J., Liang D., Zhang X.A., Liao M.A., Lv X.L., Deng Q.X., Xia H. Comparison of the fruit volatile profiles of five muscadine grape cultivars (Vitis rotundifolia Michx.) using HS-SPME-GC/MS combined with multivariate statistical analysis. Front. Plant Sci. 2021;12 doi: 10.3389/fpls.2021.728891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão MdS., Narain N., Santos MdSPd, Nunes M.L. Volatile compounds and descriptive odor attributes in umbu (Spondias tuberosa) fruits during maturation. Food Res. Int. 2011;44:1919–1926. doi: 10.1016/j.foodres.2011.01.020. [DOI] [Google Scholar]
- Gao H.Y., Zhu H.L., Shao Y., Chen A.J., Lu C.W., Zhu B.Z., Luo Y.B. Lycopene accumulation affects the biosynthesis of some carotenoid-related volatiles independent of ethylene in tomato. J. Integr. Plant Biol. 2008;50:991–996. doi: 10.1111/j.1744-7909.2008.00685.x. [DOI] [PubMed] [Google Scholar]
- Hadi M.A.M.E., Zhang F.J., Wu F.F., Zhou C.H., Tao J. Advances in fruit aroma volatile research. Molecules. 2013;18:8200–8229. doi: 10.3390/molecules18078200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jing G.X., Li T.T., Qu H.X., Yun Z., Jia Y.X., Zheng X.L., Jiang Y.M. Carotenoids and volatile profiles of yellow- and red-fleshed papaya fruit in relation to the expression of carotenoid cleavage dioxygenase genes. Postharvest Biol. Technol. 2015;109:114–119. doi: 10.1016/j.postharvbio.2015.06.006. [DOI] [Google Scholar]
- Lan T., Gao C.X., Yuan Q.Y., Wang J.Q., Zhang H.X., Sun X.Y., Lei Y.S., Ma T.T. Analysis of the aroma chemical composition of commonly planted kiwifruit cultivars in China. Foods. 2021;10:1645. doi: 10.3390/foods10071645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewinsohn E., Sitrit Y., Bar E., Azulay Y., Ibdah M., Meir A., Yosef E., Zamir D., Tadmor Y. Not just colors——carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005;16:407–415. doi: 10.1016/j.tifs.2005.04.004. [DOI] [Google Scholar]
- Lewinsohn E., Sitrit Y., Bar E., Azulay Y., Meir A., Zamir D., Tadmor Y. Carotenoid pigmentation affects the volatile composition of tomato and watermelon fruits, as revealed by comparative genetic analyses. J. Agric. Food Chem. 2005;53:3142–3148. doi: 10.1021/jf047927t. [DOI] [PubMed] [Google Scholar]
- Li C.Y., Xu Y.Y., Wu H.M., Zhao R.R., Wang X.W., Wang F.F., Fu Q.Q., Tang T.T., Shi X.W., Wang B. Flavor characterization of native Xinjiang flat peaches based on constructing aroma fingerprinting and stoichiometry analysis. Foods. 2023;12:2554. doi: 10.3390/foods12132554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C.H., Yan F.H., Gao H.J., He M., Wang Z., Cheng Y.J., Deng X.X., Xu J. Features of citrus terpenoid production as revealed by carotenoid, limonoid and aroma profiles of two pummelos (Citrus maxima) with different flesh color. J. Sci. Food Agric. 2015;95:111–119. doi: 10.1002/jsfa.6689. [DOI] [PubMed] [Google Scholar]
- Liu H.R., Cao X.M., Azam M., Wang C.F., Liu C.X., Qiao Y.J., Zhang B. Metabolism of carotenoids and β-ionone are mediated by carotenogenic genes and PpCCD4 under ultraviolet b irradiation and during fruit ripening. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.814677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W.J., Zhang Y.Y., Ma R.J., Yu M.L. Comparison of aroma trait of the white-fleshed peach ‘Hu Jing Mi Lu’ and the yellow-fleshed peach ‘Jin Yuan’ based on odor activity value and odor characteristics. Horticulturae. 2022;8:245. doi: 10.3390/horticulturae8030245. [DOI] [Google Scholar]
- Lv Y.H., Chen G.G., Ouyang H., Sang Y.Y., Jiang Y., Cheng S.B. Effects of 1-MCP treatment on volatile compounds and quality in Xiaobai apricot during storage at low temperature. J. Food Process. Preserv. 2021;45 doi: 10.1111/jfpp.15452. [DOI] [Google Scholar]
- Miyazaki T., Plotto A., Goodner K., Gmitter Jr FG. Distribution of aroma volatile compounds in tangerine hybrids and proposed inheritance. J. Sci. Food Agric. 2011;91:449–460. doi: 10.1002/jsfa.4205. [DOI] [PubMed] [Google Scholar]
- Noh Y.M., Hida A.A., Raymond O., Comte G., Bendahmane M. The scent of roses, a bouquet of fragrance diversity. J. Exp. Bot. 2024;75:1252–1264. doi: 10.1093/jxb/erad470. [DOI] [PubMed] [Google Scholar]
- Olbricht K., Grafe C., Weiss K., Ulrich D. Inheritance of aroma compounds in a model population of Fragaria×ananassa Duch. Plant Breed. 2008;127:87–93. doi: 10.1111/j.1439-0523.2007.01422.x. [DOI] [Google Scholar]
- Rey-Serra P., Mnejja M., Monfort A. Inheritance of esters and other volatile compounds responsible for the fruity aroma in strawberry. Front. Plant Sci. 2022;13 doi: 10.3389/fpls.2022.959155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J.A., Horvat R.J., Lyon B.G., Meredith F.I., Senter S.D., Okie W.R. Comparison of quality characteristics of selected yellow- and white-fleshed peach cultivars. J. Food Sci. 1990;55:1308–1311. doi: 10.1111/j.1365-2621.1990.tb03922.x. [DOI] [Google Scholar]
- Schwab W., Davidovich-Rikanati R., Lewinsohn E. Biosynthesis of plant-derived flavor compounds. Plant J. 2008;54:712–732. doi: 10.1111/j.1365-313X.2008.03446.x. [DOI] [PubMed] [Google Scholar]
- Sun X.X., Yu Y.M., Saleh A.S.M., Yang X.Y., Ma J.L., Gao Z.W., Zhang D.Q., Li W.H., Wang Z.Y. Characterization of aroma profiles of Chinese four most famous traditional red-cooked chickens using GC-MS, GC-IMS, and E-nose. Food Res. Int. 2023;173 doi: 10.1016/j.foodres.2023.113335. [DOI] [PubMed] [Google Scholar]
- Vogel J.T., Tieman D.M., Sims C.A., Odabasi A.Z., Clark D.G., Klee H.J. Carotenoid content impacts flavor acceptability in tomato (Solanum lycopersicum) J. Sci. Food Agric. 2010;90:2233–2240. doi: 10.1002/jsfa.4076. [DOI] [PubMed] [Google Scholar]
- Wang G.Z., Wang G.X., Liang L.S., Ma Q.H. Recent progress in research on the composition and synthesis of aroma volatiles in peach fruits. Food Sci. (N. Y.) 2014;35:278–284. doi: 10.7506/spkx1002-6630-201417053. [DOI] [Google Scholar]
- Xi W.P., Zhang L.N., Liu S.Y., Zhao G.H. The genes of CYP, ZEP, and CCD1/4 play an important role in controlling carotenoid and aroma volatile apocarotenoid accumulation of apricot fruit. Front. Plant Sci. 2020;11 doi: 10.3389/fpls.2020.607715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xin R., Liu X.H., Wei C.Y., Yang C., Liu H.R., Cao X.M., Wu D., Zhang B., Chen K.S. E-nose and GC-MS reveal a difference in the volatile profiles of white- and red-fleshed peach fruit. Sensors. 2018;18:765. doi: 10.3390/s18030765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X.D., Xu R., Jia Q., Feng T., Huang Q.R., Ho C.T., Song S.Q. Identification of dihydro-β-ionone as a key aroma compound in addition to C8 ketones and alcohols in Volvariella volvacea mushroom. Food Chem. 2019;293:333–339. doi: 10.1016/j.foodchem.2019.05.004. [DOI] [PubMed] [Google Scholar]
- Yu M.L., Ma R.J., Xu J.L., Shen Z.J., Song H.F., Cai Z.X., Zhang Y.Y., Zhang B.B., Du P. A new late-ripening peach cultivar ‘Xiahui 8’. Acta Hortic. Sin. 2014;41:593–594. doi: 10.16420/j.issn.0513-353x.2014.03.020. [DOI] [Google Scholar]
- Zeng Y.L., Wang M.Y., Hunter D.C., Matich A.J., McAtee P.A., Knäbel M., Hamiaux C., Popowski E.A., Jaeger S.R., Nieuwenhuizen N.J., Yauk Y.K., Atkinson R.G. Sensory-directed genetic and biochemical characterization of volatile terpene production in kiwifruit. Plant Physiol. 2020;183:51–66. doi: 10.1104/pp.20.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X.N., Su M.S., Zhou H.J., Leng F., Du J.H., Li X.W., Zhang M.H., Hu Y., Gao Y., Ye Z.W. Effect of 1-methylcyclopropene on flat peach fruit quality based on electronic senses, LC-MS, and HS-SPME-GC-MS during shelf storage. LWT - Food Sci. Technol. (Lebensmittel-Wissenschaft -Technol.) 2023;173 doi: 10.1016/j.lwt.2022.114388. [DOI] [Google Scholar]
- Zhang Y.Y., Liu W.J., Zhang B.B., Zhang Y.Y., Cai Z.X., Song H.F., Ma R.J., Yu M.L. Analysis of volatile compounds and their potential regulators in four high-quality peach (Prunus persica L.) cultivars with unique aromas. LWT--Food Sci. Technol. 2022;160 doi: 10.1016/j.lwt.2022.113195. [DOI] [Google Scholar]
- Zhang Z.H., Ye W.Z., Li C., Zhou H.H., Wang C., Liu P.H., Zhou B.X., Zhao H.Q., Wang S.C., Yang J. Volatilomics-based discovery of key volatiles affecting flavor quality in tomato. Foods. 2024;13:879. doi: 10.3390/foods13060879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B.T., Sun M., Li J.Y., Su Z.W., Cai Z.X., Shen Z.J., Ma R.J., Yan J., Yu M.L. Carotenoid profiling of yellow-flesh peach fruit. Foods. 2022;11:1669. doi: 10.3390/foods11121669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorrilla-Fontanesi Y., Rambla J.L., Cabeza A., Medina J.J., Sánchez-Sevilla J.F., Valpuesta V., Botella M.A., Granell A., Amaya I. Genetic analysis of strawberry fruit aroma and identification of O-methyltransferase FaOMT as the locus controlling natural variation in mesifurane content. Plant Physiol. 2012;159:851–870. doi: 10.1104/pp.111.188318. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data will be made available on request.