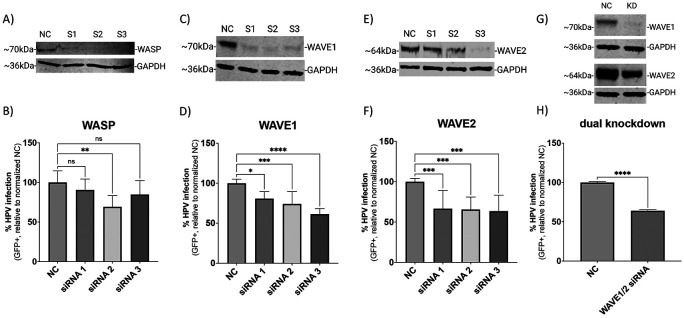
Fig 1. siRNA-mediated knockdown of WAVE1 and WAVE2 inhibits HPV16 infection in HeLa cells.
On day 0 HeLa cells were seeded and transfected with siRNA in a 6-well microplate. On day 2, cells were collected and seeded onto a 24-well microplate to establish technical replicates. On day 3, cells were infected with HPV16 PsVs (TCID30) containing a GFP reporter plasmid for 48 hours. Protein expression of relevant proteins was measured via Western blotting on day 5 (A, C, E, G). NC is the negative control siRNA used in this study, while S1, S2, and S3 refer to each of three separate siRNAs used to target the indicated proteins. For panels G and H, S2 targeting WAVE1 and S3 targeting WAVE2 were employed to achieve knockdown of both proteins. Half volumes of each siRNA were combined for transfection so that the final concentration of siRNA in each experiment remained consistent. The percentage of HPV16 infected cells was also determined on day 5 (48 hours post infection) via flow cytometry (B, D, F, H). Each bar represents three biological replicates comprised of technical triplicates and show the mean %GFP+ cells ± standard deviation (n=3, normalized to WT). 1-way ANOVA with Dunnett’s multiple comparisons test was used to statistically determine significance (ns=not significant, **p<0.001, ***p<0.0001, ****p<0.0001).