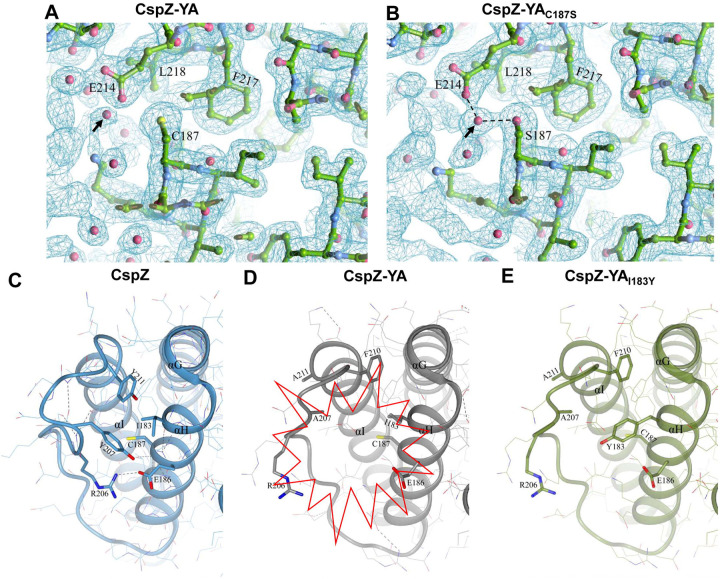
Figure 5. The comparison of CspZ, CspZ-YA, CspZ-YAC187S, and CspZ-YAI183Y structures suggest the helix H-I interactions impacted by the C187S and I183Y mutagenesis.
The structures here were obtained from CspZ (PDB ID 9F7I), CspZ-YA (PDB ID 9F1V), CspZ-YAC187S (PDB ID 9F21), and AlphaFold predicted structure of CspZ-YAI183Y. (A to B) Shown is the 2Fo-Fc electron density map contoured at 1σ of the region around C187 in (A) CspZ-YA and S187 in (B) CspZ-YAC187S. The hydrogen bond formed between the water molecule with S187 and E214 were highlighted. (C to E) The crystal structures of (C) CspZ from B. burgdorferi B31, (D) CspZ-YA, and (E) the predicted structure of CspZ-YAI183Y show the hydrophobic core accounting for helices G, H and I and the residues Y207, Y211, I183, and C187 in CspZ and the equivalent residues in CspZ-YA and CspZ-YAI183Y.