Figure 1:
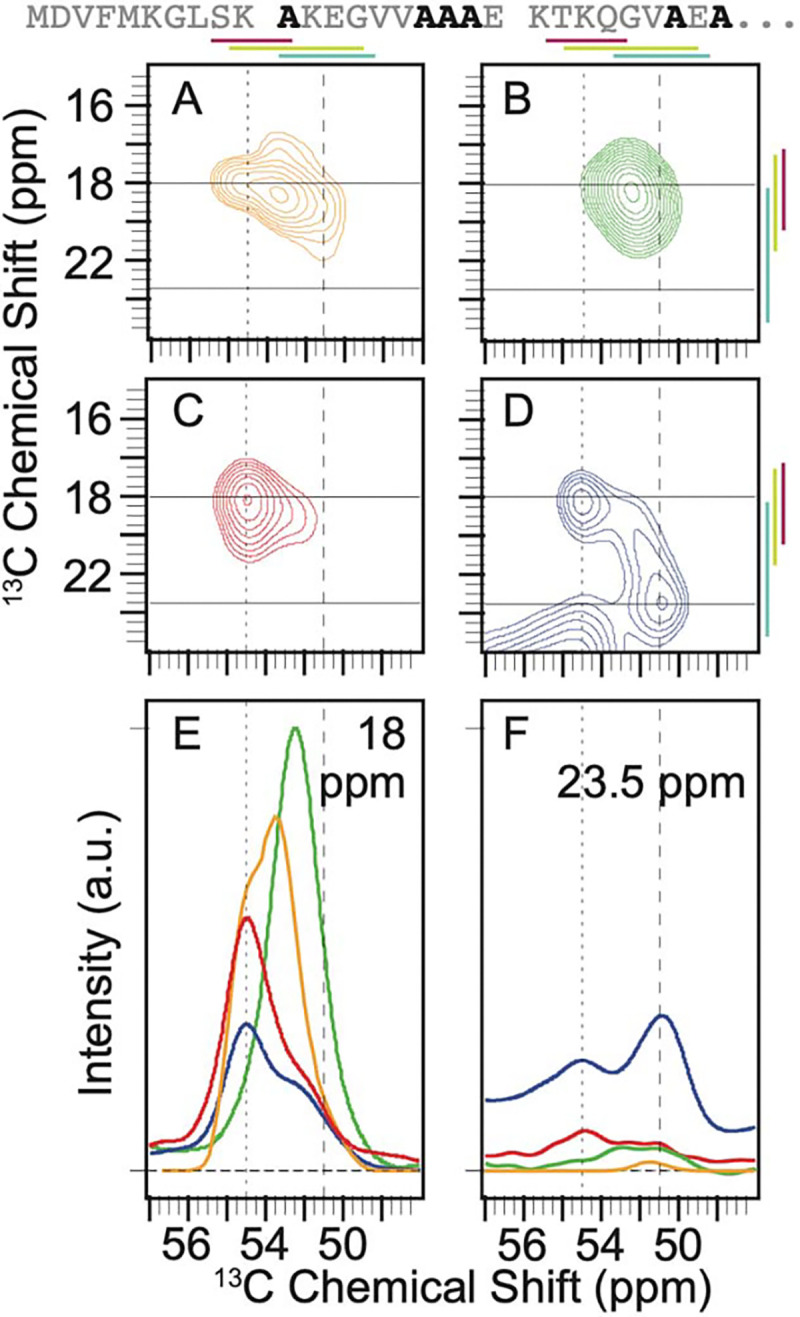
Context alters the conformational preferences of alanine residues in the amino terminal intrinsically disordered region of α-syn. The primary sequence of the isotopically labeled segment of α-syn is shown with alanine residues highlighted in black. A) The predicted peak shape for the Cα-Cβ cross peak of alanine from a statistical coil structural ensemble assuming a homogenous line width of 1.5 ppm. 13C-13C DARR spectra of frozen segmentally isotopically labeled α-syn in B) 8M urea C) in the monomeric form in buffer and D) in the amyloid fibril form in buffer. Colored bars annotate the average chemical shift ± two standard deviations for α-helices (magenta), random coils (light green) and β-strands (light blue) (45). Spectra were normalized to have the same integrated intensity. Horizontal lines at 18 ppm and 23.5 ppm mark the location of 1D slices in E and F. E) Overlay of the normalized one-dimensional slices from the 2D DARR spectra at 18 ppm. F) Overlay of normalized one-dimensional slices from the 2D DARR spectra at 23.5 ppm. Samples contained 15% d8-glycerol and 5 mM AMUPol. Spectra were collected at 600 MHz with 12 kHz MAS at 104 K.
