Abstract
Purpose
To analyze the impacts of distinct chemotherapy methods on blood lipid levels in breast cancer patients.
Methods
Three hundred breast cancer patients were selected as the research subjects. The inclusion time limit was from January 2021 to January 2023, and all received treatment in our hospital. Based on the therapy plan, the patients were divided into group A (epirubicin + cyclophosphamide followed by paclitaxel regimen, 103 premenopausal cases + 61 postmenopausal cases), group B (docetaxel + cyclophosphamide regimen, 41 premenopausal instances + 37 postmenopausal instances), group C (docetaxel + carboplatin regimen, 61 premenopausal instances + 24 postmenopausal instances), comparing the changes in blood lipid levels of patients in each group at pre-therapy and post-therapy, and the abnormality frequency of blood lipids in every group of patients after therapy.
Results
After treatment, the triglyceride (TG) levels of the three groups of patients were clearly greater than those at pre-therapy, and the high-density lipoprotein cholesterol (HDL-C) levels were clearly less than before therapy. The levels of low-density lipoprotein cholesterol (LDL-C) in group B and C patients were clearly greater than those before therapy in the same one, while the LDL-C levels in group A were clearly less than those before therapy in the same one; after therapy, the TG levels of patients in group A were clearly less than those in group B, and LDL-C, total Cholesterol (TC) levels were clearly less than that in group B and C (P < 0.05). The proportion of dyslipidemia in patients in the group A after therapy was clearly less than in group B (P < 0.05). After treatment, the HDL-C levels of premenopausal patients in the three groups were clearly less than those at pre-therapy. The TG, TC, and LDL-C levels of premenopausal patients in groups B and C were clearly greater than those at pre-therapy. The TG levels of premenopausal patients in group A were clearly less than those before therapy. After treatment, the TG and TC levels of premenopausal patients in group A were clearly less than those in group C, and the LDL-C levels were clearly less than those in group B and C (P < 0.05). The proportion of dyslipidemia in premenopausal patients in the group A and C after therapy was clearly less than the group B (P < 0.05). After therapy, the TG levels of postmenopausal patients in the three groups were clearly greater than those at pre-therapy, and the HDL-C levels were clearly less than those at pre-therapy. The LDL-C levels of postmenopausal patients in group B and C were clearly greater than those at pre-therapy. The TC and LDL-C levels of postmenopausal patients in group A were clearly less than those at pre-therapy; after therapy, the LDL-C and TC levels of postmenopausal patients in group A were clearly less than those in group B and C (P < 0.05). It had no statistically clear distinction in dyslipidemia among the three groups of postmenopausal patients after therapy (P > 0.05).
Conclusion
Chemotherapy has adverse effects on the blood lipid levels of premenopausal and postmenopausal breast carcinoma patients and increases the incidence of dyslipidemia. Compared with other regimens, epirubicin+cyclophosphamide sequential paclitaxel regimen has little impact on blood lipid level of breast carcinoma.
Keywords: chemotherapy, breast cancer, blood lipids, impact
Preface
Breast cancer is the most common female malignancy worldwide. In 2021, an estimated 284,200 new cases of breast cancer will occur and nearly 43,600 women will die from breast cancer, making it one of the leading causes of cancer death in women.1,2 Most clinical treatments include surgery, chemotherapy, and radiotherapy. Chemotherapy is one of the usual ways to treat breast carcinoma. With the rapid development of medical technology recently, the mortality ratio of breast carcinoma has been relatively reduced and the overall survival has been prolonged. Nevertheless, the frequency of other complications in breast carcinoma patients increased, leading to an increase in non tumor related mortality in breast carcinoma patients.3,4 Cardiovascular disease is a common complication of breast carcinoma, and according to statistics, the high-risk group for breast carcinoma in China is 45-year-old women, accounting for 70% of the total number. Most 45 year old women are in the perimenopausal or menopausal period, during which estrogen secretion continues to reduce and lipid metabolism is abnormal, causing an increase in the frequency of cardiovascular illness.5,6 In addition, some studies believe that cardiotoxic diseases can increase during chemotherapy treatment, which may be related to its induction of hypercholesterolemia.7,8 Therefore, analyzing the impact of different chemotherapy methods on blood lipid levels in breast carcinoma patients can help to adjust therapy plans in a timely manner, and early intervention can decrease the frequency of cardiovascular illness and have further improvement on patients prognosis. This experiment selected 300 breast carcinoma patients admitted to our hospital as the study subjects, aiming to analyze the effects of different chemotherapy methods on the blood lipid levels of breast cancer patients.
Materials and Methods
General Information
Three hundred breast carcinoma patients were selected as the research subjects, and the inclusion time limit was from January 2021 to January 2023. Inclusion criteria: ① All are breast cancer patients, and the standards refer to the “Chinese Expert Consensus on Neoadjuvant Treatment of Breast Cancer (2019 Edition)”; ② All are treated with chemotherapy in our hospital, and have a high degree of cooperation. Exclusion criteria: ① Those with previous chemotherapy, radiotherapy and other treatments; ② Those combined with other pernicious tumors; ③ Those combined with illnesses relevant to dyslipidemia; ④ Those combined with serious dysfunction of important organs. Based on the therapy plan, the patients were divided into group A (epirubicin + cyclophosphamide followed by paclitaxel regimen, 103 premenopausal cases + 61 postmenopausal cases), group B (docetaxel + cyclophosphamide regimen, 41 premenopausal instances + 37 postmenopausal instances), group C (docetaxel + carboplatin regimen, 61 premenopausal instances + 24 postmenopausal instances), it had no clear distinction in age, gender, diabetes (DM), hypertension (EH) and so on in every groups (P > 0.05). All experimental procedures were approved by the hospital ethics committee. See Table 1.
Table 1.
General Data Analysis of the Two Groups [n (%) (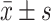 )]
)]
| grouping | n | Age (years) | BMI (kg/m2) | DM | EH |
|---|---|---|---|---|---|
| A group | 164 | 47.88±6.53 | 24.51±3.15 | 9 (5.49) | 18 (10.98) |
| B group | 71 | 47.44±6.19 | 24.48±3.19 | 5(7.04) | 10 (14.08) |
| C group | 65 | 48.10±6.53 | 24.67±2.10 | 3 (4.62) | 8(12.31) |
| x 2 /F | 0.202 | 0.087 | 0.396 | 0.461 | |
| P | 0.817 | 0.917 | 0.821 | 0.794 | |
Method
Group A was given 90 mg/m2 epirubicin, 600 mg/m2 cyclophosphamide, and 175 mg/m2 sequential paclitaxel liposome treatment, with 14 days as one cycle, and a total of 4 cycles of treatment; Group B was given the therapy of 75 mg/m2 docetaxel and 600 mg/m2 cyclophosphamide, with 21 days as one cycle, and a total of 4–6 cycles of treatment; Group C was given 75 mg/m2 docetaxel and carboplatin area under the curve (AUC) for 4–5 days, 21 days is a cycle, with a total of 4–6 cycles of treatment.
Observation Indicators
Laboratory indicators: Early morning fasting venous blood was collected from patients in all groups at pre-therapy and post-therapy, and enzyme-linked immunosorbent assay was used to detect TC, HDL-C, LDL-C and TG levels. Among them, abnormal blood lipids are TC≥5.18mmol/L, TG≥1.70mmol/L, HDL-C≤1.04 mmol/L, or LDL-C≥3.37mmol/L.
Statistical methods
SPSS20.0 software was used to make analysis of the experimental data. Taking (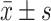 ) indicates that TG, TC, HDL-C, LDL-C and other measurement data are in line with the normal distribution. Comparison between multiple samples uses analysis of variance (F-test). When the conditions of variance analysis cannot be met, data analysis uses non-parametric testing (Kruskal–Wallis), pairwise comparisons were performed with the SNK-q test. Gender, DM, EH and other count data were expressed as (%), and the χ 2 test was used. The statistical outcomes were considered statistically obvious with P < 0.05.
) indicates that TG, TC, HDL-C, LDL-C and other measurement data are in line with the normal distribution. Comparison between multiple samples uses analysis of variance (F-test). When the conditions of variance analysis cannot be met, data analysis uses non-parametric testing (Kruskal–Wallis), pairwise comparisons were performed with the SNK-q test. Gender, DM, EH and other count data were expressed as (%), and the χ 2 test was used. The statistical outcomes were considered statistically obvious with P < 0.05.
Results
The Blood Lipid Levels Compared Between Patients in Every Groups at Pre-Therapy and Post-Therapy
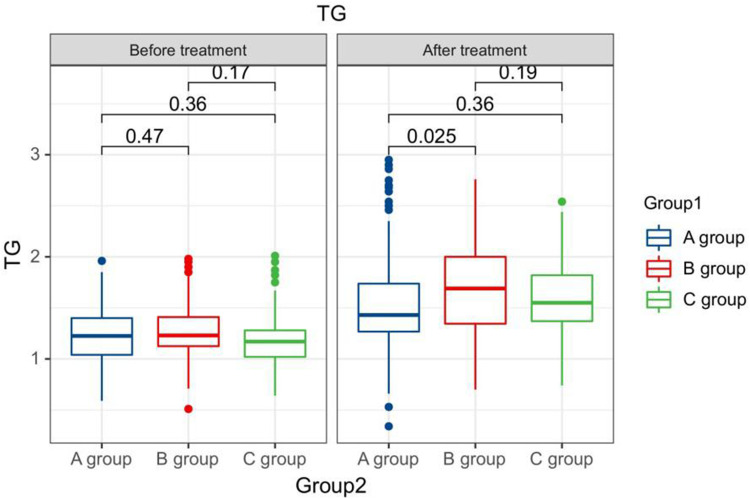
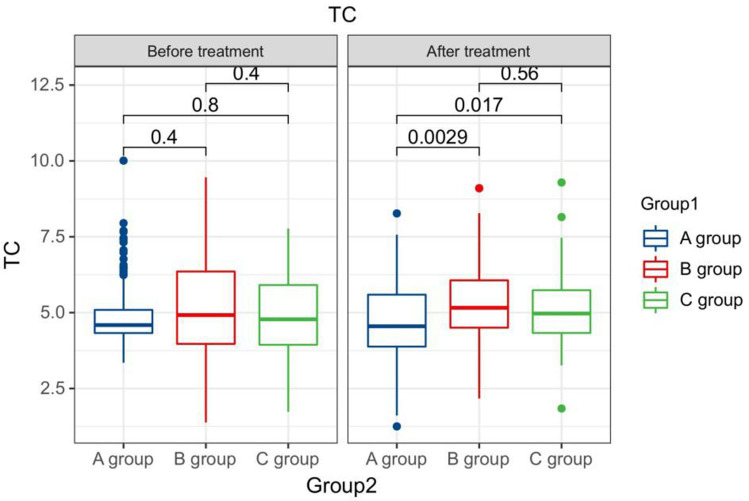
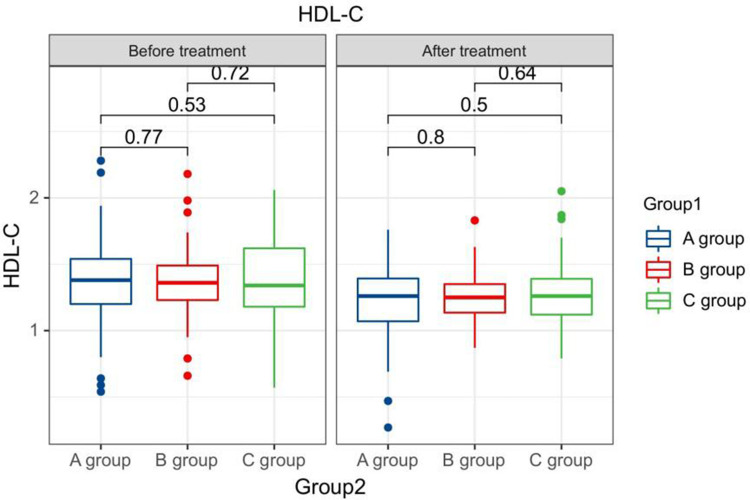
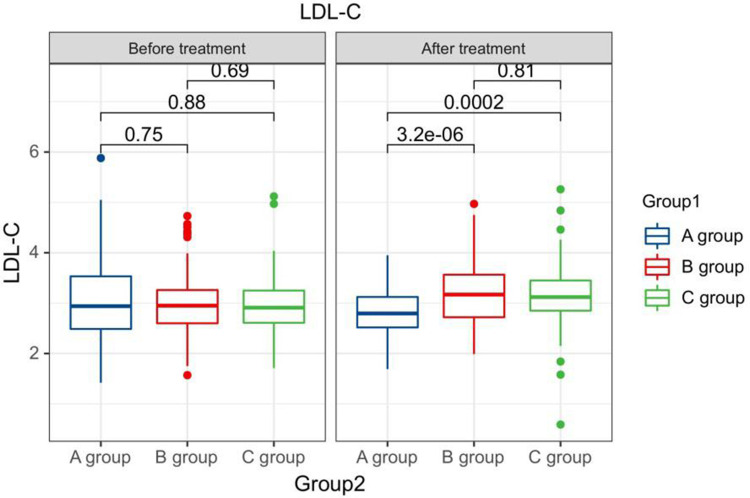
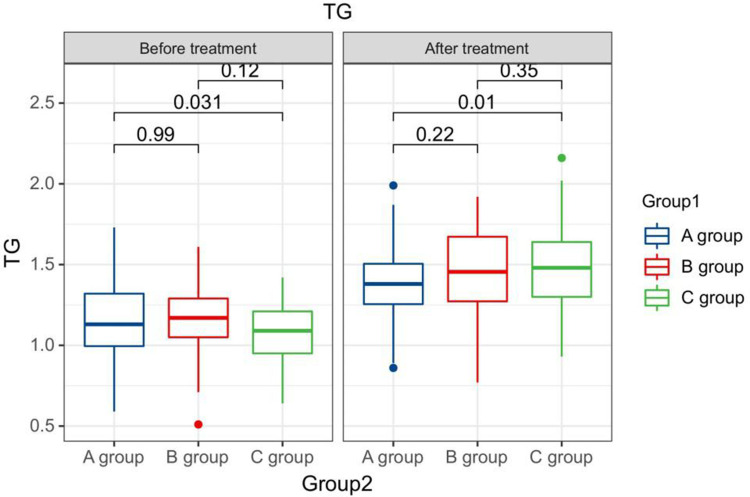
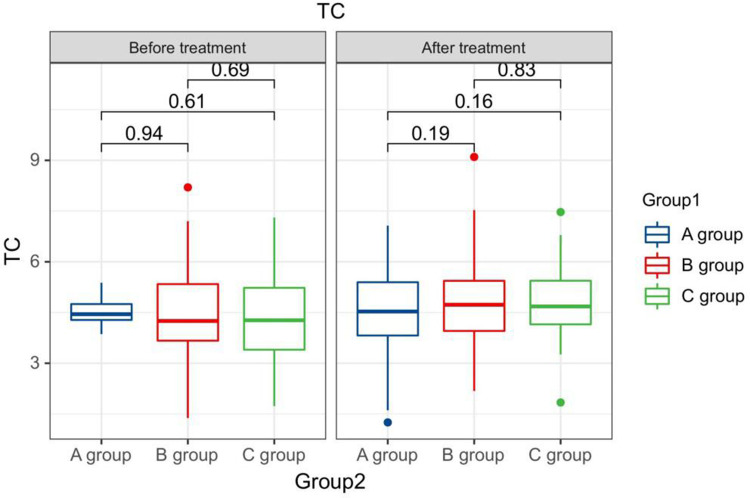
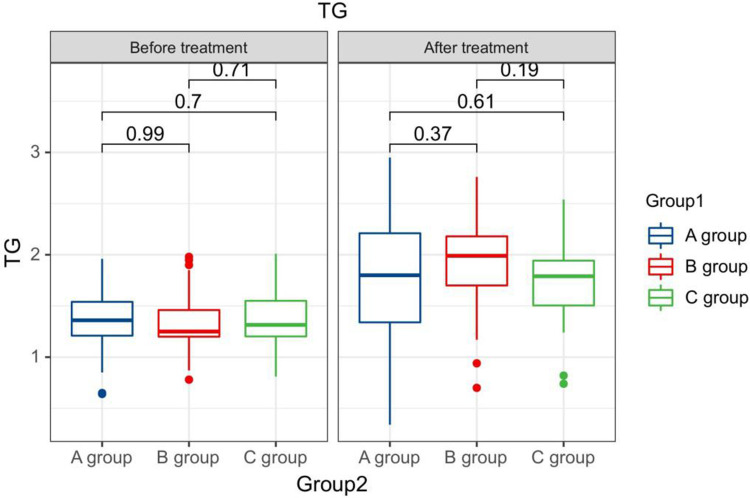
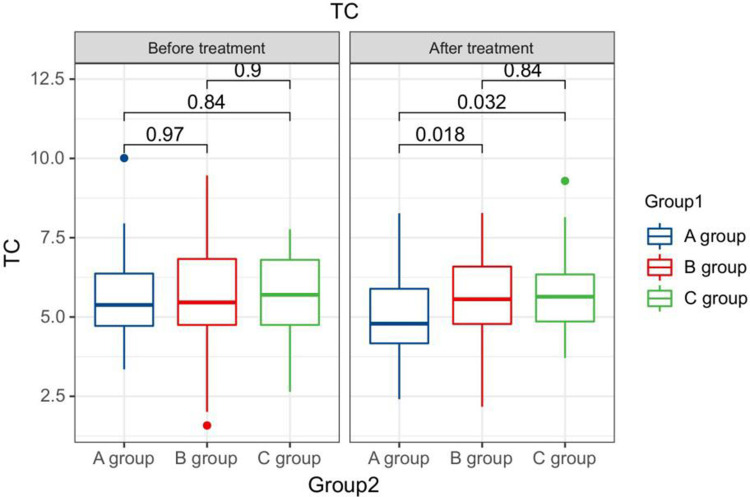
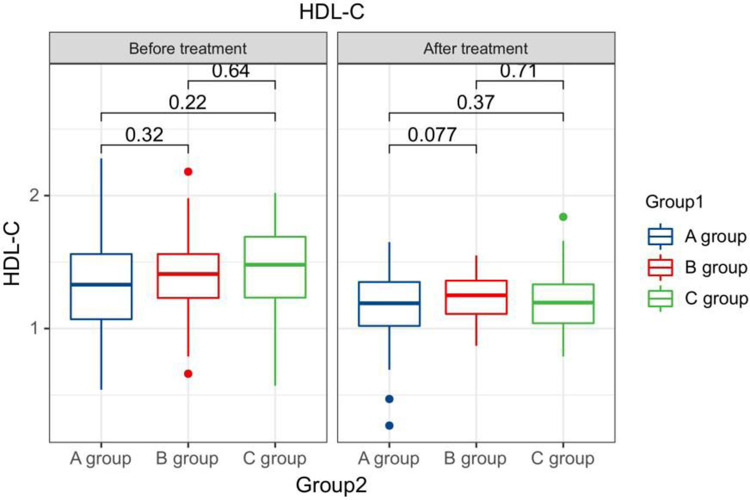
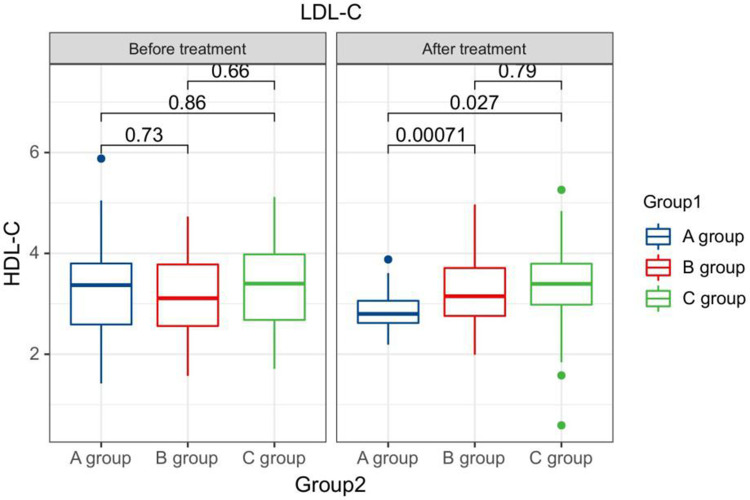
After treatment, the TG levels of the three groups of patients were clearly greater than those before therapy, and the HDL-C levels were clearly less than before therapy. The LDL-C levels of patients in group B and C were clearly greater than those before therapy. The LDL-C level in group A is clearly less than the pre-treatment level in the same one. After therapy, the TG levels of group A patients were clearly less than those of group B, and the LDL-C and TC levels of group A were clearly less than the group B and group C (P < 0.05). Seeing Table 2, Figures 1–4.
Table 2.
The Blood Lipid Levels Compared Between Sufferers in Every Groups at Pre-Therapy and Post-Therapy (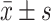 )
)
| time | Grouping | n | TG (mmol /L) | TC (mmol /L) | HDL-C (mmol /L) | LDL-C (mmol /L) |
|---|---|---|---|---|---|---|
| Before treatment | A group | 164 | 1.23±0.27 | 4.90±0.96 | 1.36±0.27 | 2.98±0.75 |
| B group | 71 | 1.26±0.24 | 5.08±1.65 | 1.37±0.24 | 3.01±0.69 | |
| C group | 65 | 1.19±0.27 | 4.85±0.83 | 1.39±0.22 | 2.96±0.38 | |
| F | 0.982 | 0.651 | 0.242 | 0.080 | ||
| P | 0.376 | 0.523 | 0.785 | 0.923 | ||
| After treatment | A group | 164 | 1.54±0.45 a | 4.68±1.33 | 1.24±0.24 a | 2.80±0.43 a |
| B group | 71 | 1.68± 0.43ab | 5.26± 1.37ab | 1.25±0.18 a | 3.18± 0.58ab | |
| C group | 65 | 1.59±0.27 a | 5.13± 0.84b | 1.27±0.18 a | 3.15± 0.19ab | |
| F | 2.690 | 5.958 | 0.274 | 17.650 | ||
| P | 0.070 | 0.003 | 0.760 | <0.001 | ||
Notes: Compare to the same one at pre-therapy, a P <0.05; compare to group A at the same time, b P <0.05.
Figure 1.
The TG levels compared between patients in every groups at pre-therapy and post-therapy.
Figure 2.
The TC levels compared between patients in every groups at pre-therapy and post-therapy.
Figure 3.
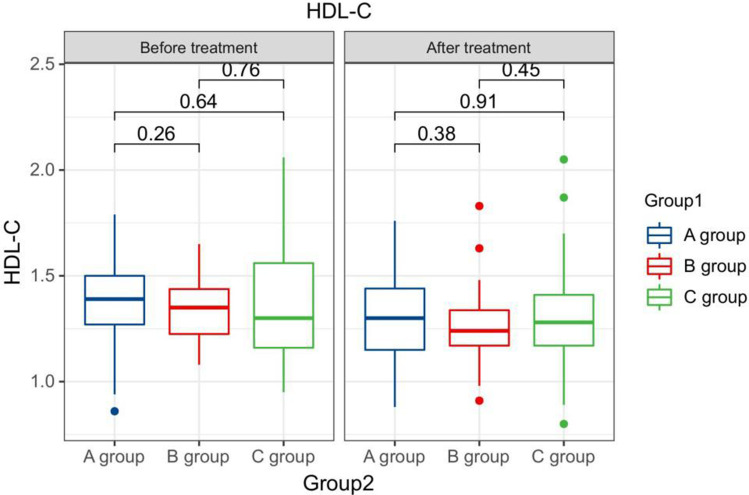
The HDL-C levels compared between patients in every groups at pre-therapy and post-therapy.
Figure 4.
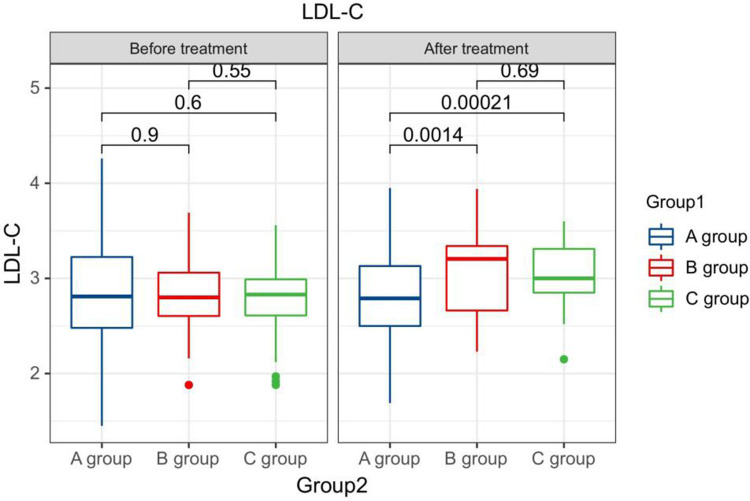
The LDL-C levels compared between patients in every groups at pre-therapy and post-therapy.
The Dyslipidemia Compared Between the 2 Groups of Patients After Therapy
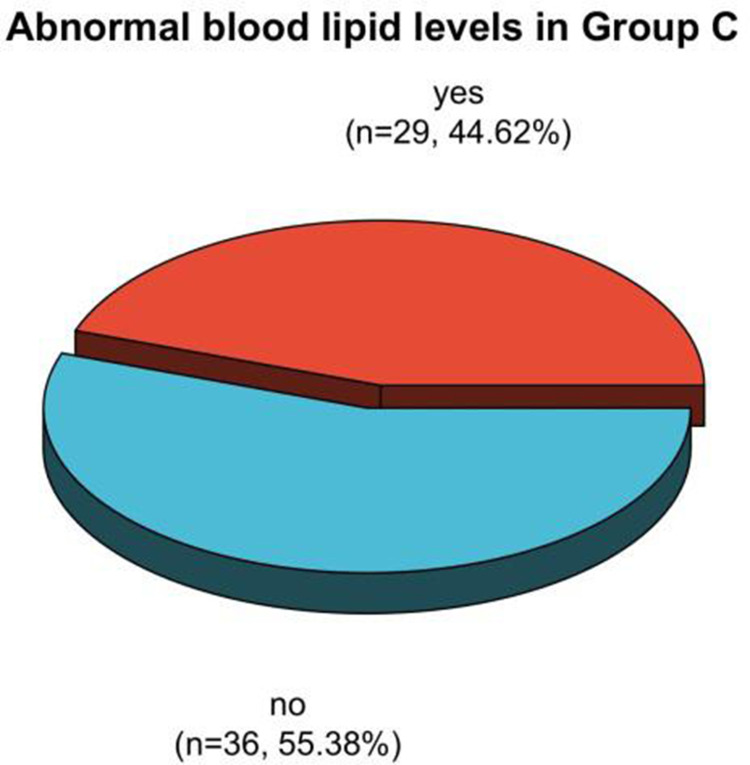
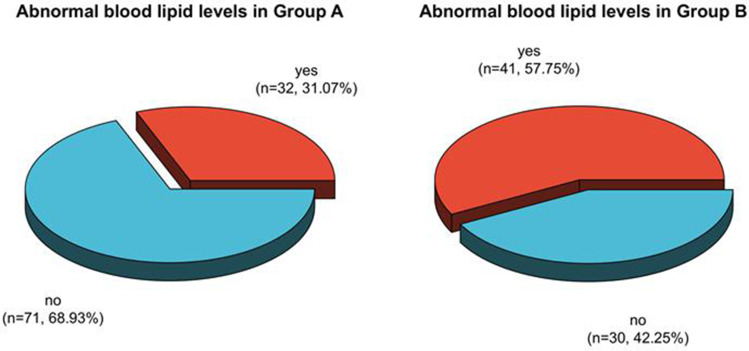
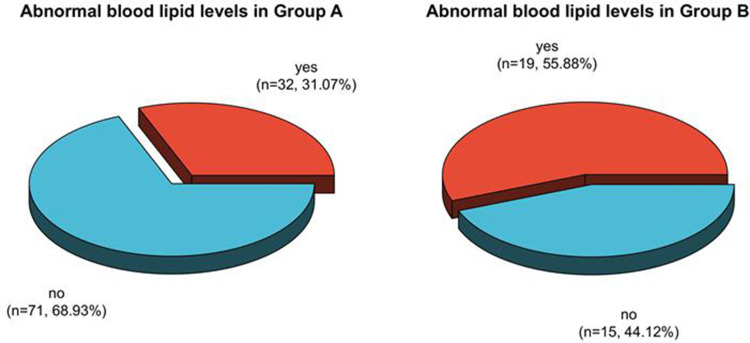
The incidence rates of dyslipidemia after therapy in patients in group A, B, and C were 36.59%, 57.75%, and 44.62%, respectively. The proportion of patients in group A with dyslipidemia after therapy was clearly less than the one in group B (P < 0.05). See Table 3, Figures 5 and 6.
Table 3.
The Dyslipidemia Compared Between the Two Groups of Sufferers After Therapy [n (%)]
| Grouping | n | Dyslipidemia | |
|---|---|---|---|
| Yes | No | ||
| A group | 164 | 60 (36.59) | 104 (63.41) |
| B group | 71 | 41(57.75) a | 30 (42.25) |
| C group | 65 | 29 (44.62) | 36 (55.38) |
| χ2 | 9.091 | ||
| P | 0.011 | ||
Note: Compare to group A, aP <0.05.
Figure 5.
Analysis of dyslipidemia in patients in Group A and B after therapy.
Figure 6.
Analysis of dyslipidemia in patients in group C after treatment.
Comparison of Blood Lipid Levels Between Two Groups of Premenopausal Patients at Pre-Therapy and Post-Therapy After therapy, the HDL-C levels in the three groups of premenopausal patients were clearly less than those in the same group at pre-therapy. The TG, TC, and LDL-C levels in the B and C groups of premenopausal patients were clearly greater than those in the same one before therapy, while the TG levels in the A group were clearly less than that in the same one before therapy; after therapy, the TG and TC levels of premenopausal patients in group A were clearly less than those in group C, and the LDL-C levels were clearly less than that in group B and C (P < 0.05). See Table 4, Figures 7–10.
Table 4.
The Blood Lipid Levels Compared Between Two Groups of Premenopausal Sufferers at Pre-Therapy and Post-Therapy (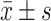 )
)
| Time | Grouping | n | TG (mmol /L) | TC (mmol /L) | HDL-C (mmol /L) | LDL-C (mmol /L) |
|---|---|---|---|---|---|---|
| Before treatment | A group | 103 | 1.16±0.23 | 4.50±0.31 | 1.38±0.19 | 2.83±0.57 |
| B group | 34 | 1.16±0.24 | 4.52±1.46 | 1.34±0.15 | 2.84±0.39 | |
| C group | 41 | 1.08±0.16 | 4.39±1.35 | 1.36±0.27 | 2.78±0.41 | |
| F | 1.841 | 0.247 | 0.474 | 0.142 | ||
| P | 0.162 | 0.781 | 0.623 | 0.868 | ||
| After treatment | A group | 103 | 1.39±0.21 a | 4.50±1.26 | 1.29±0.20 a | 2.79±0.46 |
| B group | 34 | 1.45±0.27 a | 4.85±1.37 a | 1.26±0.18 a | 3.09± 0.45ab | |
| C group | 41 | 1.51± 0.26ab | 4.79± 1.05ab | 1.30±0.25 | 3.05± 0.33ab | |
| F | 4.110 | 4.158 | 0.359 | 9.217 | ||
| P | 0.018 | 0.017 | 0.699 | <0.001 | ||
Note: Compare to the same one before therapy, a P <0.05; compare to group A at the same time, b P <0.05.
Figure 7.
The TG levels compared in premenopausal patients in every groups at pre-therapy and post-therapy.
Figure 8.
The TC levels compared in premenopausal patients in every groups at pre-therapy and post-therapy.
Figure 9.
Comparison of HDL-C levels in premenopausal patients in every groups at pre-therapy and post-therapy.
Figure 10.
Comparison of LDL-C levels in premenopausal patients in every groups at pre-therapy and post-therapy.
Comparison of Dyslipidemia in Two Groups of Premenopausal Patients After Therapy
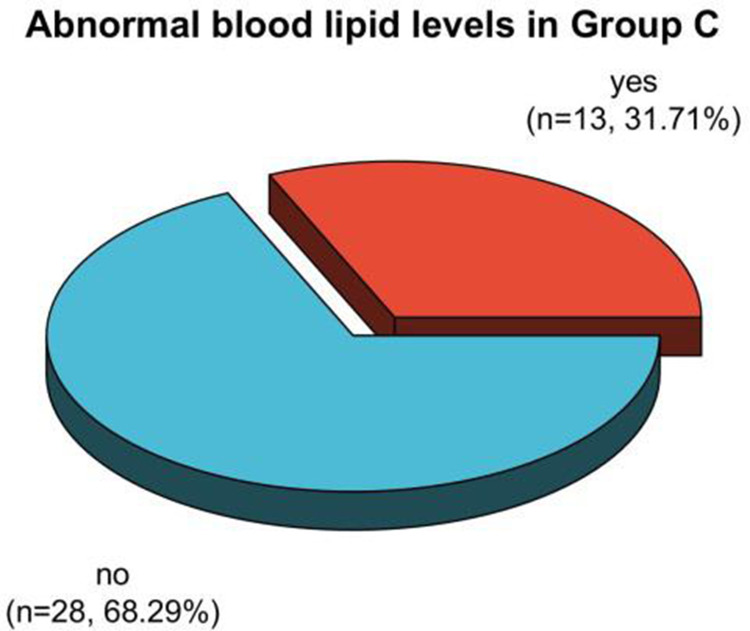
The frequency ratios of dyslipidemia in premenopausal patients in Group A, B, and C after therapy were 31.07%, 55.88%, and 31.71%, respectively. The proportion of dyslipidemia in premenopausal patients in Group A and C after therapy was clearly less than in Group B (P < 0.05). See Table 5, Figures 11 and 12.
Table 5.
The Dyslipidemia Compared in Two Groups of Premenopausal Sufferers After Therapy [n (%)]
| Grouping | n | Dyslipidemia | |
|---|---|---|---|
| yes | yes | ||
| A group | 103 | 32 (31.07) | 71 (68.93) |
| B group | 34 | 19 (55.88) a | 15 (44.12) |
| C group | 41 | 13 (31.71) b | 28 (68.29) |
| χ2 | 7.253 | ||
| P | 0.027 | ||
Note: Compare to A group, a P <0.05; compare to B group, b P <0.05.
Figure 11.
Analysis of dyslipidemia in premenopausal patients in Group A and B after therapy.
Figure 12.
Analysis of dyslipidemia in premenopausal patients in Group C after treatment.
The Blood Lipid Levels Compared Between Two Groups of Postmenopausal Patients at Pre-Therapy and Post-Therapy
After therapy, the TG levels of postmenopausal patients in the three groups were clearly greater than those before therapy in the same group, and the HDL-C levels were clearly less than before therapy in the same group. The LDL-C levels of postmenopausal patients in group B and C were clearly greater than those before therapy in the same one. The TC and LDL-C levels of postmenopausal patients in group A were clearly less than those before therapy in the same one; after therapy, the LDL-C and TC levels of postmenopausal patients in the A group were clearly less than the group B and C (P < 0.05). See Table 6, Figures 13–16.
Table 6.
The Blood Lipid Levels Compared Between Every Groups of Sufferers at Pre-Therapy and Post-Therapy (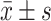 )
)
| Time | Grouping | n | TG (mmol /L) | TC (mmol /L) | HDL-C (mmol /L) | LDL-C (mmol /L) |
|---|---|---|---|---|---|---|
| Before treatment | A group | 61 | 1.35±0.28 | 5.58±1.27 | 1.33±0.36 | 3.23±0.94 |
| B group | 37 | 1.35±0.29 | 5.59±1.67 | 1.40±0.30 | 3.17±0.85 | |
| C group | twenty four | 1.38±0.33 | 5.64±1.32 | 1.44±0.38 | 3.27±0.91 | |
| F | 0.100 | 0.017 | 1.021 | 0.101 | ||
| P | 0.905 | 0.983 | 0.363 | 0.904 | ||
| After treatment | A group | 61 | 1.79± 0.61a | 4.98±1.41 a | 1.16± 0.27a | 2.82±0.39 a |
| B group | 37 | 1.89±0.45 a | 5.64± 1.27b | 1.24±0.18 a | 3.26± 0.67b | |
| C group | twenty four | 1.73±0.46 a | 5.71± 1.36b | 1.22±0.26 a | 3.32± 1.01b | |
| F | 0.697 | 3.967 | 1.39 | 7.983 | ||
| P | 0.500 | 0.021 | 0.253 | <0.001 | ||
Note: Compare to the same one before therapy, a P <0.05; compare to group A at the same time, b P <0.05.
Figure 13.
The TG levels in postmenopausal patients compared between the each group at pre-therapy and post-therapy.
Figure 14.
Comparison of TC levels in postmenopausal patients in every groups at pre-therapy and post-therapy.
Figure 15.
Comparison of HDL-C levels in postmenopausal patients in every groups at pre-therapy and post-therapy.
Figure 16.
Comparison of LDL-C levels in postmenopausal patients in every groups at pre-therapy and post-therapy.
Comparison of Dyslipidemia in Two Groups of Postmenopausal Patients After Therapy
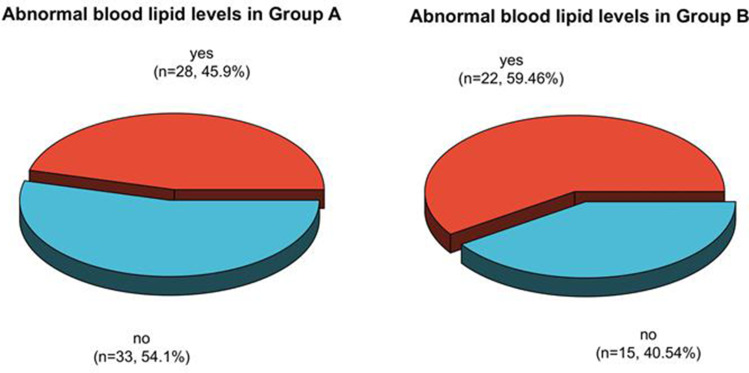
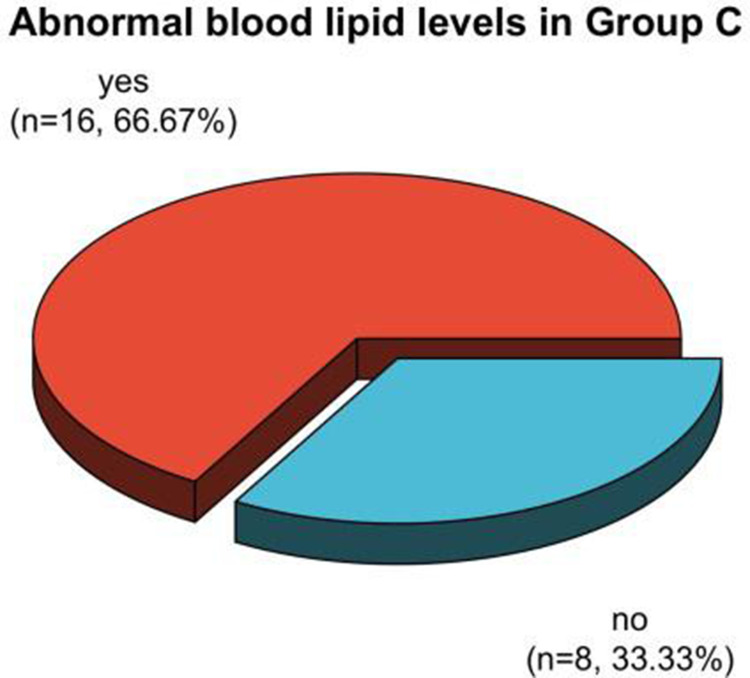
The frequency ratios of dyslipidemia in postmenopausal patients in Group A, B, and C after therapy were 45.90%, 59.46%, and 66.67%, respectively. It had no statistically clear distinction in dyslipidemia in the three groups after therapy (P > 0.05). See Table 7, Figures 17 and 18.
Table 7.
Comparison of Dyslipidemia Between Two Groups of Postmenopausal Patients After Treatment [n (%)]
| Grouping | n | Dyslipidemia | |
|---|---|---|---|
| Yes | Yes | ||
| A group | 61 | 28 (45.90) | 33 (54.10) |
| B group | 37 | 22 (59.46) | 15 (40.54) |
| C group | twenty four | 16 (66.67) | 8(33.33) |
| χ2 | 3.605 | ||
| P | 0.165 | ||
Figure 17.
Analysis of dyslipidemia in postmenopausal patients in Group A and B after therapy.
Figure 18.
Analysis of dyslipidemia in postmenopausal patients in Group C after treatment.
Discussion
Breast carcinoma is one of the most usual female malignancies in the world and the leading cause of most carcinoma-relevant deaths.9,10 With the increase in life pressure and changes in dietary structure recently, the frequency of breast carcinoma has increased year after year, and is trending younger.11,12 Breast cancer-related research has also become the focus of scholars in the medical field.
Chemotherapy is a common way to treat breast cancer and has a vital function in the treatment process. It can help extend the illness-free survival and overall survival of breast carcinoma patients.9,10 Nevertheless, with the introduction of new therapy methods and the continuous increase in life expectancy, it is necessary to recognize the long-term and delayed consequences of carcinoma therapy. Chemotherapy drugs could not only kill tumor cells but also affect other normal cells, causing various adverse reactions and side effects,11,12 and some researches believed that chemotherapy drugs could lead to abnormal blood lipid levels in the body.13,14 In this research, the TG levels among the three groups of patients after therapy were clearly greater than those before therapy of the same group, and the HDL-C levels were clearly less than the one in the same group before therapy. The proportion of dyslipidemia in the group A after therapy was clearly less than the group B. It shows that chemotherapy treatment can affect the body’s blood lipid levels and lead to dyslipidemia in breast cancer patients. The reason is that chemotherapy treatment can reduce fat consumption while killing tumor cells, and endothelial dysfunction and insulin resistance are aggravated during chemotherapy, which could help promote the synthesis of blood lipids.15,16 What’s more, chemotherapy can induce fatigue and other adverse reactions, leading to a decrease in physical activity, while drug treatment such as glucocorticoids can increase the patient’s appetite, ultimately leading to an increase in blood lipids.17,18 Cardiovascular diseases such as heart failure, myocardial ischemia and hypertension are common complications after chemotherapy for breast cancer, and hyperlipidemia is one of the high-risk factors for cardiovascular disease, increasing the incidence of cardiovascular illness in breast carcinoma patients and affecting the prognosis of patients,19,20 early analysis of the impact of different chemotherapy methods on blood lipid levels in breast cancer can help guide treatment plans and improve patient prognosis.
Endothelial dysfunction can occur during chemotherapy treatment and increase insulin resistance, thus increasing patients’ blood lipid levels. Anthracyclines and taxanes are common drugs currently used to treat breast carcinoma. Anthracyclines are represented by epirubicin, and taxanes are represented by paclitaxel.21,22 Studies have shown that compared with non-anthracyclines, anthracyclines have little effect on the body’s blood lipids, and taxanes themselves have little effect on blood lipids.23,24 However, glucocorticoids need to be treated before taxanes are used, and glucocorticoids could cause water and sodium retention, abnormal glucose and lipid metabolism, and increase the level of blood lipids.25,26 The epirubicin + cyclophosphamide followed by paclitaxel regimen, the docetaxel + cyclophosphamide regimen, and the docetaxel + carboplatin regimen are all common clinical regimens for the therapy of breast carcinoma. This research selected breast carcinoma as the research object and compared the effects of three chemotherapy methods on breast carcinoma. The comparison showing that after therapy, the TG and TC levels in group A premenopausal patients were clearly less than those in group C, and the levels of LDL-C were clearly less than those in group B and C. The proportion of dyslipidemia in premenopausal patients in Group A and C after treatment was clearly less than the one in B group. After treatment, the LDL-C and TC levels of postmenopausal patients in group A were clearly less than the group B and C. It had no statistically clear distinction in dyslipidemia among the three groups of postmenopausal patients after treatment. This shows that the epirubicin + cyclophosphamide followed by paclitaxel regimen has a relatively small effect on the blood lipid levels of breast carcinoma patients. The reason for this is that anthracycline drugs themselves have little impact on body blood lipids, and in the sequential paclitaxel regimen of epirubicin+cyclophosphamide, paclitaxel liposomes are used as paclitaxel drugs, which do not require the use of glucocorticoids, thus reducing the impact on blood lipids to a certain extent.
In summary, chemotherapy treatment has adverse effects on the blood lipid levels of premenopausal and postmenopausal breast carcinoma patients and increases the incidence of dyslipidemia. Compared with other regimens, epirubicin+cyclophosphamide sequential paclitaxel regimen has little impact on blood lipid level of breast carcinoma. Nevertheless, due to the small number of samples in this study and the samples coming from patients in our hospital, the experimental results may be random. In addition, due to the short research period of this study, the risk of long-term cardiovascular events in breast cancer caused by different chemotherapy methods has not yet been analyzed. In the future, it will be the research direction for in-depth exploration.
Funding Statement
There is no funding to report.
Data Sharing Statement
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the 1964 helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Ethics Committee of Civil Aviation General Hospital. Written informed consent was obtained.
Consent for Publication
Informed consent was obtained from all individual participants included in the study.
Author Contributions
Guarantor of integrity of the entire study: Jiaqi Mu; study concepts & design: Gangjun Jiao; definition of intellectual content: Mai Zhou; literature research, data acquisition and analysis: Jiaqi Mu; manuscript preparation & editing: Jiaqi Mu. All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Wilkinson L, Gathani T. Understanding breast cancer as a global health concern. Brit J Radiol. 2022;95(1130):20211033. doi: 10.1259/bjr.20211033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kadamkulam Syriac A, Nandu NS, Leone JP. Central nervous system metastases from triple-negative breast cancer: current treatments and future prospective. Breast Cancer. 2022;14:1–13. doi: 10.2147/BCTT.S274514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang Y, Zhang H, Song X, Yang Q. Metastatic heterogeneity of breast cancer: molecular mechanism and potential therapeutic targets. Semi Cancer Biol. 2020;60:14–27. doi: 10.1016/j.semcancer.2019.08.012 [DOI] [PubMed] [Google Scholar]
- 4.Xu X, Zhang M, Xu F, Jiang S. Wnt signaling in breast cancer: biological mechanisms, challenges and opportunities. Mol Cancer. 2020;19(1):165. doi: 10.1186/s12943-020-01276-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang H, Mao X. Evaluation of the efficacy of neoadjuvant chemotherapy for breast cancer. Drug Des Devel Ther. 2020;14:2423–2433. doi: 10.2147/DDDT.S253961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Murto MO, Simolin N, Arponen O, et al. Statin use, cholesterol level, and mortality among females with breast cancer. JAMA Network Open. 2023;6(11):e2343861. doi: 10.1001/jamanetworkopen.2023.43861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montemurro F, Nuzzolese I, Ponzone R. Neoadjuvant or adjuvant chemotherapy in early breast cancer ? Expert Opin Pharmacother. 2020;21(9):1071–1082. doi: 10.1080/14656566.2020.1746273 [DOI] [PubMed] [Google Scholar]
- 8.Stankowski-Drengler TJ, Livingston- Rosanoff D, Schumacher JR, Hanlon BM, Hitchcock ME, Neuman HB. Breast cancer outcomes of neoadjuvant versus adjuvant chemotherapy by receptor subtype: a scoping review. J Surg Res. 2020;254:83–90. doi: 10.1016/j.jss.2020.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Miglietta F, Bottosso M, Griguolo G, Dieci MV, Guarneri V. Major advancements in metastatic breast cancer treatment: when expanding options means prolonging survival. ESMO open. 2022;7(2):100409. doi: 10.1016/j.esmoop.2022.100409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Silvestrini VC, Thomé CH, Albuquerque D, et al. Proteomics analysis reveals the role of ubiquitin specific protease (USP47) in epithelial to mesenchymal transition (EMT) induced by TGFβ2 in breast cells. J Proteomics. 2020;219:103734. doi: 10.1016/j.jprot.2020.103734 [DOI] [PubMed] [Google Scholar]
- 11.Barzaman K, Karami J, Zarei Z, et al. Breast cancer: biology, biomarkers, and treatments. Int Immunopharmacol. 2020;84:106535. doi: 10.1016/j.intimp.2020.106535 [DOI] [PubMed] [Google Scholar]
- 12.Feng J, Symonds EA, Karnes JH. Visualization and quantification of the association between breast cancer and cholesterol in the all of us research program. Cancer Inf. 2023;22:11769351221144132. doi: 10.1177/11769351221144132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Amerizadeh A, Vaseghi G, Farajzadegan Z, Asgary S. An updated systematic review and meta-analysis on association of serum lipid profile with risk of breast cancer incidence. Int J Preventive Med. 2022;13(1):142. doi: 10.4103/ijpvm.IJPVM_285_20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lofterød T, Frydenberg H, Veierød MB, et al. The influence of metabolic factors and ethnicity on breast cancer risk, treatment and survival: the Oslo ethnic breast cancer study. Acta oncologica. 2022;61(5):649–657. doi: 10.1080/0284186X.2022.2053573 [DOI] [PubMed] [Google Scholar]
- 15.Nouri M, Mohsenpour MA, Katsiki N, et al. Effect of serum lipid profile on the risk of breast cancer: systematic review and meta-analysis of 1,628,871 women. J Clin Med. 2022;11(15):4503. doi: 10.3390/jcm11154503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campos AL, Sawada MIBAC, Santana MFM, et al. The increased antioxidant action of HDL is independent of HDL cholesterol plasma levels in triple-negative breast cancer. Front Oncol. 2023;13:1111094. doi: 10.3389/fonc.2023.1111094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Loosen SH, Kostev K, Luedde M, Luedde T, Roderburg C. Low blood levels of high-density lipoprotein (HDL) cholesterol are positively associated with cancer. J Cancer Res Clin Oncol. 2022;148(11):3039–3046. doi: 10.1007/s00432-021-03867-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wu J, Lei X, Pan X, Zeng X, Li W. Association between serum lipids and breast cancer risk in premenopausal women: systematic review and meta-analysis. Int J Med Res. 2021;49(11):3000605211061033. doi: 10.1177/03000605211061033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gupta A, Saraiya V, Deveaux A, et al. Association of lipid profile biomarkers with breast cancer by molecular subtype: analysis of the MEND study. Sci Rep. 2022;12(1):10631. doi: 10.1038/s41598-022-13740-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alimperti A, Alikari V, Tsironi M, et al. Lipid disturbances in breast cancer patients during chemotherapy. Nurs Rep. 2023;13(4):1500–1510. doi: 10.3390/nursrep13040126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lu Q, Wu X, Zhu Y, et al. Effects of chemotherapy on serum lipids in Chinese postoperative breast cancer patients. Cancer Manage Res. 2020;12:8397–8408. doi: 10.2147/CMAR.S253397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dong S, Yu J, Chen X, Shen K. Association of serum lipid levels and clinical outcomes in early breast cancer patients. Therapeut Adv Med Oncol. 2023;15:17588359231177004. doi: 10.1177/17588359231177004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qu F, Chen R, Peng Y, et al. Assessment of the predictive role of serum lipid profiles in breast cancer patients receiving neoadjuvant chemotherapy. J Breast Cancer. 2020;23(3):246–258. doi: 10.4048/jbc.2020.23.e32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang F, de Bock GH, Denig P, Landman GW, Zhang Q, Sidorenkov G. Role of serum lipids, blood glucose and blood pressure in breast cancer risk for women with Type 2 diabetes mellitus. Clin Epidemiol. 2023;15:109–121. doi: 10.2147/CLEP.S386471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sun XB, Liu WW, Wang B, et al. Correlations between serum lipid and Ki-67 levels in different breast cancer molecular subcategories. Oncol Lett. 2022;25(2):53. doi: 10.3892/ol.2022.13639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sawada MIBAC, de Fátima Mello Santana M, Reis M, et al. Increased plasma lipids in triple-negative breast cancer and impairment in HDL functionality in advanced stages of tumors. Sci Rep. 2023;13(1):8998. doi: 10.1038/s41598-023-35764-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The simulation experiment data used to support the findings of this study are available from the corresponding author upon request.