Abstract
Backgrounds
The rapid expansion of an intraparenchymal hematoma following cerebral contusion often results in high mortality rates and a poor prognosis. Effective tools are essential for predicting and monitoring the incidence of traumatic intraparenchymal hematoma (tICH) and identifying patients at high risk of tICH expansion. This enables timely surgical interventions and appropriate medical management. Recently, numerous novel predictive tools have been developed and employed to predict tICH progression. Therefore, this review aims to outline the latest advancements in predicting tICH expansion.
Methods
To find relevant studies, a search was conducted on PubMed and Google Scholar for articles published from January 2020 to April 2024. The search string used was (Cerebral Contusion) AND (Intraparenchymal Hematoma Progression OR Parenchymal Hematoma Expansion OR Intracerebral Hemorrhage Progression) AND (Predictor or Forecasting Tool).
Results
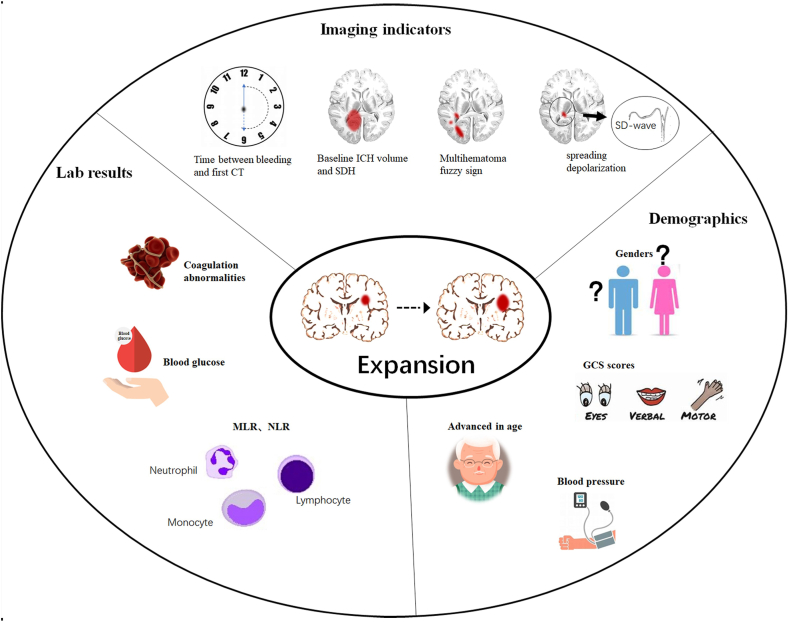
In this narrative review, we focused on various radiological, clinical, and innovative indicators of acute tICH progression that have been developed and/or validated in recently years. Additionally, we explore the impact of tICH progression on long-term outcomes, suggesting potential avenues for future research. The spread of depolarization in the cortex could be a key factor in forecasting and controlling the growth of tICH in the times ahead.
Conclusions
This study offers a comprehensive look at various cutting-edge imaging predictors, inflammatory indices, and integrated predictors that can aid neurosurgeons in categorizing, handling, and treating high-risk patients with acute tICH expansion.
Keywords: Hematoma expansion, Hematoma progression, Predictors, Multihematoma fuzzy sign, Cerebral contusion
Highlights
-
•
This review provides an all-around overview of predicting acute tICH expansion.
1. Introduction
Traumatic brain injury refers to brain dysfunction caused by external forces, and has become a leading cause of disability and death among young adults worldwide. Each year, TBI results in approximately 10 million hospitalizations or fatalities [1,2]. There are several pathological characteristics of TBI, including axonal and hemorrhagic injuries, as well as dynamic changes in pathophysiology, which contribute to the variability of the condition. This sequence of events is expected to result in gradual bleeding, with cerebral contusions being particularly susceptible to progressive hemorrhage (see Fig. 1).
Fig. 1.
Acute traumatic intraparenchymal hematoma expansion (tICH) and its clinical and radiological predictors.
Cerebral contusion is a common primary brain injury caused by external force, resulting in bleeding and/or crushing of the superficial brain cortex with the dura mater intact. It mainly occurs when the brain tissue undergoes linear acceleration or deceleration, or rotational movement within the skull after external force, leading to collision or friction between the brain surface and the inner surface of the skull or base of the skull. During the acute phase of a brain contusion, there is often an increase in intraparenchymal hematoma, known as traumatic intraparenchymal hematoma (tICH) expansion. This severe clinical deterioration often leads to increased morbidity and subsequent clinical decline. Generally, acute tICH expansion is identified by hematoma growth on baseline computed tomography (CT), which typically occurs within 24 h and rarely progresses after 3–4 days for most patients [3,4]. A follow-up imaging study revealed that between 16 % and 75 % of contusions exhibit progression [[4], [5], [6], [7]]. However, this variation can be attributed to the different thresholds used for identifying tICH expansion in various studies. Studies using relative volume changes to define progression have varied significantly, with some defining it as a 5 % increase in volume, whereas others require a 50 % increase [6,[8], [9], [10], [11], [12], [13]]. Conversely, absolute volume changes have been defined as a notable growth of either a 1 cm increase in diameter or a 12.5 ml volume increase [3,[14], [15], [16]]. Nevertheless, many studies have combined relative and absolute volume changes to determine an increase in tICH [[17], [18], [19], [20]]. In a recent multicenter cohort study, it was shown that absolute tICH expansion is a better predictor of traumatic brain injury prognosis compared to relative expansion [21]. Various methods are employed to measure the volume of tICH, including traditional ABC/2 methods and automatic or semiautomatic measurement methods based on imaging software. Although the ABC/2 method has been commonly used in previous studies [[22], [23], [24], [25]], there is a growing trend toward using automatic or semiautomatic tICH volume measurement methods due to the need for increased measurement accuracy [25,26].
Despite the ongoing controversy surrounding the association between tICH expansion and prognosis in patients with brain contusions, it is clear that tICH expansion indicates a significant worsening of the patient's condition in the acute phase. Hence, it is essential to recognize high-risk patients for intracerebral hematoma expansion in the initial phases of cerebral contusion, as they may require early surgical intervention or intensified medical management such as surgery, intubation, ventilation, and neurological monitoring.
Early tICH expansion is crucial for patient classification and personalized care for those with brain contusions [27]. However, there is currently a scarcity of reliable tools for accurately predicting tICH expansion in clinical practice. Over the years, the range of indicators for predicting tICH expansion has expanded, encompassing traditional imaging markers, laboratory parameters, and integrated prediction models [14,20,28,29]. This narrative review aims to discuss the phenomenon of contusion progression, novel mechanisms associated with tICH expansion, and a variety of novel predictors for acute tICH expansion.
2. Methodology
2.1. Literature search strategy
In our research, we reviewed conventional imaging markers, laboratory parameters, and combined prediction models for acute traumatic intracerebral hemorrhage expansion in individuals with primary cerebral contusion. The author (G. C.) conducted a search for relevant articles on PubMed and Google Scholar from January 2020 to April 2024. The search terms included “Cerebral Contusion,” “Intraparenchymal Hematoma Progression” or “Parenchymal Hematoma Expansion” or “Intracerebral Hemorrhage Progression,” and “Predictor” or “Forecasting Tool.” Relevant articles were identified by screening their titles and abstracts. The references cited in each publication were reviewed to find relevant information for the current study. Articles with a high number of citations in the past 5 years were selected as focal points, and their references were examined. If there was a conflict regarding the inclusion of a manuscript, the other author (H. K.) was consulted to make a decision. The scope of this review is on innovative predictors for traumatic parenchymal hematoma expansion, not traumatic nonparenchymal hematoma expansion or spontaneous parenchymal hematoma expansion.
2.2. Literature inclusion and Exclusion Criteria
Inclusion Criteria: Only observational studies, both retrospective and prospective, were included in this study if they were solely focused on acute tICH expansion/progression. 1) The study subjects comprised individuals aged 18 years or older with primary cerebral contusion, with the exception of those who had received anticoagulant treatment. 2) The goal of the study was to establish, refine, or verify a novel predictor or combined predictive model for acute tICH expansion. 3) Original research was conducted in the study design, and 4) the expected outcome was the occurrence of acute tICH expansion/progression.
Exclusion Criteria: 1) Articles not written in Chinese or English will be excluded. 2) Reviews, conference papers, master's theses, newspaper articles, and personal opinions will not be considered for inclusion.
3. Pathophysiology of contusion progression
The pathophysiology of contusion progression involves various mechanisms. Initially, external force applied to the head leads to the rupture or damage of blood vessels [30].
The pathophysiology of hemorrhagic progression in cerebral contusion encompasses a variety of mechanisms. Initially, trauma to the head leads to blood vessel rupture or damage. After that, the inflammation response and cell damage triggered by a brain contusion will intensify the permeability of blood vessels and the destruction of the blood-brain barrier [31]. This allows red blood cells, proteins, and other cellular components to extravasate into the brain tissue, leading to further expansion of tICH. In addition, cellular damage and vascular injury caused by cerebral contusions can trigger an inflammatory response [32]. Inflammatory cells in the brain, as well as those entering from the outside, cause edema in brain tissue, increasing pressure within the hematoma and damaging surrounding normal brain tissue. Activation of specificity protein 1 and nuclear factor-ĸB upregulates sulfonylurea receptor 1, which increases blood-brain barrier (BBB) permeability and promotes edema formation [33,34]. In addition, cerebral contusion can lead to abnormalities in the coagulation system, such as changes in platelet aggregation and coagulation factor activity,35which may also contribute to tICH enlargement. Moreover, the presence of hematomas and reduced blood supply after cerebral contusions cause hypoxia and ischemia in brain tissues [36], which further lead to cell death and damage. To sum up, the development of cerebral contusion hematomas is a result of various interconnected factors, such as vascular damage, heightened vascular permeability, inflammatory reactions, coagulation abnormalities, and hypoxia-ischemia. Through their combined effects, these mechanisms drive the formation and progression of tICH, ultimately leading to the malignant deterioration of brain function.
4. Imaging predictors
The significance of imaging examinations in identifying brain contusions and detecting tICH expansion cannot be overstated. In this discussion, we will focus on the latest indications for using imaging to predict tICH expansion.
Several studies and reviews have emphasized the crucial role of noncontrast computed tomography (NCCT) imaging in predicting tICH expansion. Conventional imaging features such as subarachnoid hemorrhage (SAH), subdural hemorrhage (SDH), midline shift, contrast extravasation (CE), and skull fracture have proven to be effective predictors of tICH expansion [37].
SAH leads to the leakage of breakdown products from erythrocytes and cerebral vasospasm, which ultimately results in ischemia and necrosis of the blood vessel wall. This process can trigger an inflammatory cascade following the extravasation of red blood cells [4,38,39]. SDH might be a sign of bridging veins and venous sinus damage, which can lead to continuous bleeding, increased edema, and compression effects [4,27]. Skull fractures may damage meningeal arteries and veins, which can lead to persistent bleeding. Venous sinus rupture, plate barrier and middle meningeal artery hemorrhage caused by skull fractures can eventually lead to tICH expansion.38EDH can promote tICH expansion by causing arteriovenous hemorrhage, further damaging the underlying cortex, and the compressive effects on the hematoma. In addition, a midline shift of more than 5 mm can also be a reliable indicator of potential expansion of a traumatic intracranial hemorrhage.
A variety of factors contribute to midline shift, including swelling, vasospasm, laceration, contusion, endothelial cells or microvascular injury [[39], [40], [41]].
4.1. Multihemotoma fuzzy sign
Recently, several studies have proposed the use of the multihemotoma fuzzy sign as an indicator of post-contusion brain injury [19,27]. Identified by the presence of multiple blurred areas on the NCCT scan, this sign is a dependable indicator for predicting the early significant growth of intraparenchymal hematomas in cerebral contusion. The multihematoma fuzzy sign is defined as 1) if three or more adjacent high-density hematomas are found in the area of contusion and the distance between them is not greater than the diameter of the largest hematoma, 2) if relatively low-density fuzzy signals are present in the area surrounding these high-density hematomas, and 3) if the contrast between the high-density hematomas and low-density fuzzy areas is not less than 20 Hounsfield units. In order to be recognized as a multihematoma fuzzy sign, multiple adjacent hematomas must meet all three of these criteria. Nonetheless, it is worth noting that the multihematoma fuzzy sign should not be attributed to the existence of multiple hematomas that are divided by regions of relatively low-density gray/white matter [19]. The presence of the multihematoma fuzzy sign in the region of cerebral contusion typically suggests the simultaneous existence of a blood clot (seen as a high-density shadow on NCCT) and fresh liquid blood (seen as a low-density shadow on NCCT). If fresh liquid blood is present, it usually indicates that bleeding is happening. Multiple studies have verified that individuals who exhibit a mixed density sign following a cerebral contusion have a greater chance of experiencing acute tICH expansion. Even though there are imaging predictors available for early prediction, such as contrast extravasation (CE) on contrast CT scans, these predictors may not be widely accessible or routinely performed in emergency settings for traumatic brain injury patients. Moreover, the use of CTA can increase patients' radiation exposure. On the other hand, the NCCT predictor of the multihematomal fuzzy sign is more readily available in clinical settings and offers a cost-effective advantage compared to other predictors, such as baseline CT volume, time to baseline CT, and multiple hematomas. This method achieves a nice equilibrium between precision and ease of use.
To sum up, the NCCT sign, especially the multihematomal fuzzy sign, is a helpful tool in guiding the timely and categorized treatment of individuals with brain contusions. For example, a study analyzing the correlation between multiple hematoma fuzzy signs and the severity of injury (measured by the Glasgow Coma Scale) in brain contusion patients found a significant correlation [19]. This indicates that for patients with moderate to severe brain contusions, the presence of multiple hematoma fuzzy signs on NCCT images should prompt healthcare professionals to enhance medical management or take preemptive measures to prevent hematoma expansion.
4.2. Contrast extravasation (CE)
Despite the fact that contrast extravasation (CE) scans are not frequently utilized for the detection of brain contusions in comparison to NCCT, there have been several studies examining the use of CE scans in predicting tICH expansion.
The extravasation sign or CE in enhanced CT images is a crucial predictor of tICH expansion [42,43]. CE suggests that damage to cerebrovascular endothelial cells could happen in TBI patients, leading to active bleeding [43,44]. CE was initially used to predict hematoma expansion and poor prognosis in patients with spontaneous intracerebral hematoma (ICH). Recent studies have shown that CE can predict the development of tICH after a traumatic brain injury, which is why CE was included in the analysis of this review [33,45].
Mechanistically, a traumatic penumbra develops around the site of initial injury following a brain concussion. Metabolic function within the penumbra area becomes disrupted, rendering the brain tissue more vulnerable to secondary harm. Post-brain trauma, cells in the penumbra zone trigger specific proteins like NF-ĸB and specificity protein 1, which in turn cause alterations in the expression of Aquaporin 4 and Sulfur Receptor 1. This shift heightens the permeability of the BBB, leading to angio-edema, endothelial cell death, capillary rupture, and ultimately leading to contrast agent seepage.
4.3. Radiomics analysis
Radiomics is an emerging technology capable of extracting subtle and discriminable information from medical images, such as NCCT images, and converting it into high-dimensional quantitative features. Radiomics provides a distinct advantage in capturing image details beyond human observation, including texture and high-level characteristics. These features can then be analyzed using statistical methods and mathematical algorithms to identify the most relevant quantitative features for clinical diagnosis. The general process for predicting tICH expansion using radiomics can be summarized as follows: First, 2D or 3D CT images of all selected patients were obtained, and the images were standardized. Second, image features are extracted from the regions of interest using software packages based on Python, and then filters such as wavelet, Laplacian of Gaussian, and local binary pattern filters are applied to these images. During this process, numerous features related to shape, first-order, and texture aspects are extracted. Third, statistical methods such as intraclass correlation and logistic regression were utilized to preliminarily select specific radiomic features, and then the feature set was refined using the least absolute shrinkage and selection operator method. Finally, the accuracy and reliability of these radiomic feature sets were assessed using deep machine learning to predict tICH expansion through either internal dataset cross-validation or external dataset validation [[46], [47], [48]].
Radiomics has proven to be an effective and powerful tool commonly used in the central nervous system, especially in tumors [[49], [50], [51], [52]], and for predicting hematoma growth in hypertensive intracerebral hemorrhage patients [53,54]. Multiple recent studies have shown the feasibility of radiomics in predicting tICH expansion, with the accuracy of composite predictive models or nomograms that combine traditional clinical predictors with radiomics surpassing existing clinical and imaging indicators [[1], [2], [3]].
5. Inflammatory predictors
5.1. Monocytes to lymphocytes ratio (MLR)
The MLR upon admission is a simple indicator that can predict the development of acute intraparenchymal bleeding after brain contusion [17]. It has been utilized as a predictor in various contexts, such as cancer and cardiovascular disease. Although its effectiveness in predicting acute tICH expansion is still uncertain, this study demonstrated a robust link between the MLR and the acute tICH expansion in cerebral contusions. The nomogram created from the MLR model has been shown to be easily accessible and user-friendly [17].
Moreover, neuroinflammation, an essential characteristic of acute traumatic intracerebral hemorrhage (tICH) resulting from cerebral contusion [55], is recognized to have a significant impact on the progression of tICH expansion. When brain contusion occurs, the BBB weakens, allowing inflammatory cells such as monocytes, neutrophils, and lymphocytes to infiltrate the brain, exacerbating damage. Monocytes have been shown to aid neutrophil infiltration, leading to an unfavorable prognosis. Moreover, decreased lymphocyte levels may be associated with the expansion of spontaneous intraparenchymal hematomas and worse clinical outcomes [56,57].
MLR serves as a marker for the equilibrium between innate and adaptive immunity, providing valuable information on inflammation levels. Studies have confirmed its role as an independent risk factor for acute tICH expansion and unfavorable long-term outcomes. Therefore, by detecting high-risk patients promptly, MLR can facilitate more efficient treatment, decrease the necessity for repeat CT scans and intensive monitoring, and enhance the care of individuals with cerebral contusion [4].
5.2. Neutrophil to lymphocyte ratio (NLR)
The NLR demonstrates a favorable outlook in forecasting the prognosis of patients with severe traumatic brain injury and spontaneous intracerebral hemorrhage. By using standard laboratory tests, one can easily establish this ratio, ensuring objectivity, accessibility, cost-effectiveness, and reproducibility.
TBI-induced alterations contribute to inflammation, endothelial cell activation, and increased levels of adhesion factors and inflammatory cytokines, which accelerate the progression of neurodegeneration and neuroinflammation. Due to the release of inflammatory cytokines, proteases, and metalloproteinases, along with the activation of endothelial cells, the permeability of the blood-brain barrier is enhanced. Neutrophils are among the initial white blood cells that migrate into the central nervous system through meningeal blood vessels and transcellular pathways. Moreover, there was a significant elevation in neutrophil levels in the bloodstream within 48 h following TBI. Findings have revealed that peripheral macrophages are instrumental in fostering the proliferation of T lymphocytes after TBI. This process is essential for repairing damaged brain tissues as these macrophages release growth factors and help regulate functions [58].
The NLR has been proposed as a tool for evaluating inflammation in individuals with various brain conditions, including gliomas, stroke, and epilepticus [[59], [60], [61], [62]]. Studies have shown that in cases of severe tICH, a higher NLR at admission is linked to poor functional outcomes and mortality within one year. The predictive abilities of the NLR upon admission and the Glasgow Coma Scale (GCS) score for severe TBI were compared by Chen et al. [63] By conducting receiver operating characteristic analysis, the NLR was evaluated and found to have an AUC of 0.723. At a threshold of 13.05, the predicted sensitivity was 60.2 % and specificity was 71.1 %.
Despite ongoing progress in critical care, accurately predicting the initial development of traumatic intracranial hemorrhage (tICH) remains challenging. Zhuang et al. indicated that the NLR could be a useful tool in anticipating the initial emergence of tICH in individuals suffering from traumatic brain injury [18]. Three statistical models were utilized to assess the relationship between the NLR and tICH: a basic multivariate model that did not include the NLR, a comprehensive model that incorporated the NLR, and a NLR indicator. The study revealed that NLR exhibited strong predictive accuracy for early tICH expansion, with an accuracy of 82 %, outperforming the basic model's accuracy of 80 % and demonstrating consistent predictive abilities.
6. Combinatorial predictor (nomogram)
The identification of multiple hematomas on noncontrast CT scans, the MLR at admission, and the NLR has demonstrated great efficacy in predicting the development of traumatic intracerebral hematoma following brain bruising. However, there is still a need for a comprehensive model that incorporates these crucial predictors to further enhance the predictive accuracy. Sheng et al. proposed a comprehensive model named traumatic parenchymatous hematoma expansion aid (TPHEA), which integrates clinical and imaging factors [27]. The primary predictors in this model were a fuzzy sign of multihematoma, initial volume of tICH, and the MLR inflammatory index upon admission. Additionally, Zhu et al. reported a similar combined nomogram for tICH expansion that included the presence of SDH, age, presence of multiple hematoma fuzzy signs, coagulopathy, initial hematoma volume, and the localization of hematoma [64]. These comprehensive models are extremely accurate in predicting various patient subgroups, enabling high-risk populations to receive cost-effective, safe treatments like hematoma clearance, recombinant activated factor VII therapy, and diligent monitoring. Conversely, low-risk patients have the option to skip unnecessary CT scans and rigorous monitoring. These complex predictive models or nomograms may offer personalized care and treatment for patients with cerebral contusion. However, it is important to note that many comprehensive prediction tools, such as nomograms, are based on retrospective data sets. This could lead to an overestimation or underestimation of the actual rate of acute tICH expansion, highlighting the need for further validation of their clinical applicability.
7. Other clinical predictors
Research has consistently demonstrated that age is a contributing factor to the likelihood of tICH expansion in elderly TBI patients, possibly due to the increased rigidity and fragility of blood vessels as individuals age. Furthermore, older patients are more susceptible to hypertension, diabetes mellitus, and other conditions that affect vascular structural lesions, as well as coagulation disorders, all of which may contribute to the occurrence of tICH expansion. The impact of sex on tICH expansion remains controversial. Some scholars support the association between sex and tICH expansion, suggesting that female estrogen and progesterone have a neuroprotective effect. However, other studies have pointed out that women who experience trauma tend to be more sensitive to anxiety, depression, and other negative emotions than men are, which can also adversely affect their prognosis [65].
Higher systolic blood pressure on admission is associated with tICH expansion. However, recent studies have suggested that a history of hypertension and overall blood pressure levels after TBI are strongly associated with tICH expansion. Additionally, a history of hypertension and hospitalization may be more significant than a single blood pressure measurement [66]. Chronic essential hypertension can lead to vascular remodeling and endothelial dysfunction, resulting in increased blood‒brain barrier permeability and elevated systolic blood pressure due to heightened intracranial pressure following TBI. This can increase the pressure on intracranial capillaries, increasing the risk of cerebral hemorrhage and edema. There have also been reports indicating a significantly lower hospital admission rate for patients with TBI than for those admitted for other reasons.
Furthermore, elevated glucose levels exceeding 10 mmol/L upon admission have been associated with increased tICH expansion risk and prognosis impact, although the exact mechanism requires further investigation.
The incidence of coagulation disorders after TBI ranges from 40 % to 80 %. Coagulation disorders are considered important risk factors and independent predictors of tICH expansion [67]. Various coagulation markers associated with tICH expansion include international normalized ratio, prothrombin time, partial thromboplastin time, activated partial thromboplastin time, and platelet count. While, the associations between different coagulation indices and tICH incidence, as well as their predictive value, remain controversial.
7.1. The perspective of tICH expansion: spreading depolarization
In a recent study, a mouse model of spontaneous cerebral hemorrhage was used to demonstrate that hematoma expansion triggers spreading depolarization (SD), suggesting that SD could be used to detect intracranial hematoma expansion [68]. However, there are currently no relevant reports on the involvement of SD in studies of hematoma expansion within traumatic brain parenchyma. Prolonged spontaneous depolarization waves have been shown to have negative effects on ischemic stroke, aneurysmal subarachnoid hemorrhage, and TBI [69,70]. In normal brains, the hemodynamic response to SD is transient vasodilation and hyperemia, followed by severe vasoconstriction and hypoperfusion (inverse coupling) in traumatic brain injury [[66], [71], [72]]. After brain injury, the presence of SD leads to the reversal of glutamate transport and uncoupling of the neurovascular unit, causing microcirculatory disturbances and exacerbating brain injury. The constriction of blood vessels around the damaged area reduces blood flow and oxygen supply, whereas damage to the blood‒brain barrier worsens swelling and harm to nearby brain cells [68].
Furthermore, SD can induce neuroinflammation and oxidative stress, which can worsen brain injury and may be related to hematoma expansion [73].
Given that hematoma expansion can continue for several hours after symptom onset and can be observed during hospitalization [74,75], SDs may not only have the potential to predict acute intraparenchymal hematoma expansion in traumatic brain injury patients but also serve as a therapeutic target [[76], [77], [78]]. Further research is needed to determine whether similar findings apply to humans and other brain regions, as the study primarily focused on the cerebral cortex.
8. Limitation
Readers should be mindful of certain limitations when interpreting this study. Firstly, the majority of new predictive factors are developed based on retrospective observational cohorts. Due to the inherent bias in retrospective data, the accuracy of these predictive factors may require further validation in multicenter prospective cohorts. Secondly, in constructing predictive models, certain continuous variables may be converted into categorical variables, such as age, which, while enhancing the convenience of using predictive models, may diminish the model's performance. Thirdly, a significant portion of research studies fail to offer detailed information on data loss or exclusions in the methodology section, resulting in a decrease in the available sample size and selection bias. Finally, all predictive models mentioned in this study for acute tICH expansion are just tools to assist neurosurgeons in evaluating hematoma enlargement in patients with brain contusions. These predictive tools cannot replace the professional judgment of doctors. When a particular predictive model indicates an increased risk of acute tICH expansion in a patient, the next step of whether intervention measures are needed, what kind of intervention measures to take, such as enhancing monitoring or surgically removing the hematoma, all require the attending physician to make a comprehensive judgment based on other physiological indicators of the patient to provide the final plan. It is crucial for clinicians and policymakers to consider reviews like this study early on when selecting which clinical predictors to endorse in evidence-based guidelines or incorporate into practice.
9. Conclusions
Acute intraparenchymal hematoma expansion after cerebral contusion is a frequent and perilous outcome that poses a substantial risk of hospitalization and mortality. Recognizing individuals with a high likelihood of acute tICH expansion is essential, as they necessitate immediate surgical intervention and individualized care. This study provides an overview of several innovative imaging predictors, inflammatory indices, and integrated predictors. These new indicators can assist neurosurgeons in classifying, managing, and treating patients with brain contusions. Recent findings have indicated that the propagation of depolarization in the cortex could be a significant factor in forecasting and addressing the expansion of tICH. While, further studies are needed to fully understand its impact on acute tICH expansion in humans.
CRediT authorship contribution statement
Gengyu Chen: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Huibin Kang: Writing – review & editing, Writing – original draft, Supervision, Conceptualization.
The accessibility of information and resources
All data that was produced or examined during this study has been incorporated into this published article (along with its supplementary information files).
Funding
Not applicable.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Not applicable.
Abbreviation List
- BBB
blood‒brain barrier
- CE
contrast extravasation
- CT
computed tomography
- EDH
epidural hemorrhage
- GCS
Glasgow Coma Scale
- ICH
intracerebral hemorrhage
- MLR
The ratio of monocytes to lymphocytes
- NCCT
noncontrast computed tomography
- NLR
The Ratio of Neutrophils to Lymphocytes
- SAH
subarachnoid hemorrhage
- SD
spreading depolarization
- SDH
subdural hemorrhage
- TBI
Traumatic brain injury
- tICH
Traumatic intraparenchymal hematoma
- TPHEA
traumatic parenchymatous hematoma expansion aid
References
- 1.Maas A.I.R., Menon D.K., David Adelson P.D., et al. Traumatic brain injury: integrated approaches to improve prevention, clinical care, and research. Lancet Neurol. 2017;16(12) doi: 10.1016/S1474-4422(17)30371-X. [DOI] [PubMed] [Google Scholar]
- 2.Hyder A.A., Wunderlich C.A., Puvanachandra P., Gururaj G., Kobusingye O.C. The impact of traumatic brain injuries: a global perspective. NeuroRehabilitation. 2007;22(5) doi: 10.3233/nre-2007-22502. [DOI] [PubMed] [Google Scholar]
- 3.Yadav Y.R., Basoor A., Jain G., Nelson A. Expanding traumatic intracerebral contusion/hematoma. Neurol. India. 2006;54(4) doi: 10.4103/0028-3886.28109. [DOI] [PubMed] [Google Scholar]
- 4.Kurland D., Hong C., Aarabi B., Gerzanich V., Simard J.M. Hemorrhagic progression of a contusion after traumatic brain injury: a review. J. Neurotrauma. 2012;29(1):19–31. doi: 10.1089/neu.2011.2122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barton C.A., Oetken H.J., Hall N.L., Webb A.J., Hoops H.E., Schreiber M. Incidence of traumatic intracranial hemorrhage expansion after stable repeat head imaging: a retrospective cohort study. Am. J. Surg. 2022;224(2) doi: 10.1016/j.amjsurg.2022.01.028. [DOI] [PubMed] [Google Scholar]
- 6.Cepeda S., Gómez P.A., Castaño-Leon A.M., Munarriz P.M., Paredes I., Lagares A. Contrecoup traumatic intracerebral hemorrhage: a geometric study of the impact site and association with hemorrhagic progression. J. Neurotrauma. 2016;33(11) doi: 10.1089/neu.2015.4153. [DOI] [PubMed] [Google Scholar]
- 7.Letourneau-Guillon L., Huynh T., Jakobovic R., Milwid R., Symons S.P., Aviv R.I. Traumatic intracranial hematomas: prognostic value of contrast extravasation. Am. J. Neuroradiol. 2013;34(4) doi: 10.3174/ajnr.A3309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alahmadi H., Vachhrajani S., Cusimano M.D. The natural history of brain contusion: an analysis of radiological and clinical progression: clinical article. J. Neurosurg. 2010;112(5) doi: 10.3171/2009.5.JNS081369. [DOI] [PubMed] [Google Scholar]
- 9.Sharma M., Mittal R., Sharma A., Gandhi A. Posttraumatic contusion: clinical and radiologic factors for progression in early postinjury period. Indian Journal of Neurotrauma. 2016;13(1) doi: 10.1055/s-0036-1580717. [DOI] [Google Scholar]
- 10.White C.L., Griffith S., Caron J.L. Early progression of traumatic cerebral contusions: characterization and risk factors. J. Trauma Inj. Infect. Crit. Care. 2009;67(3) doi: 10.1097/TA.0b013e3181b2519f. [DOI] [PubMed] [Google Scholar]
- 11.Rehman L., Afzal A., Aziz H., Akbar S., Abbas A., Rizvi R. Radiological parameters to predict hemorrhagic progression of traumatic contusional brain injury. J. Neurosci. Rural Pract. 2019;10(2) doi: 10.4103/jnrp.jnrp_335_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iaccarino C., Schiavi P., Picetti E., et al. Patients with brain contusions: predictors of outcome and relationship between radiological and clinical evolution: clinical article. J. Neurosurg. 2014;120(4) doi: 10.3171/2013.12.JNS131090. [DOI] [PubMed] [Google Scholar]
- 13.Cepeda S., Gómez P.A., Castaño-Leon A.M., Martínez-Pérez R., Munarriz P.M., Lagares A. Traumatic intracerebral hemorrhage: risk factors associated with progression. J. Neurotrauma. 2015;32(16) doi: 10.1089/neu.2014.3808. [DOI] [PubMed] [Google Scholar]
- 14.Juratli T.A., Zang B., Litz R.J., et al. Early hemorrhagic progression of traumatic brain contusions: frequency, correlation with coagulation disorders, and patient outcome: a prospective study. J. Neurotrauma. 2014;31(17) doi: 10.1089/neu.2013.3241. [DOI] [PubMed] [Google Scholar]
- 15.Narayan R.K., Maas A.I.R., Servadei F., et al. Progression of traumatic intracerebral hemorrhage: a prospective observational study. J. Neurotrauma. 2008;25(6) doi: 10.1089/neu.2007.0385. [DOI] [PubMed] [Google Scholar]
- 16.Chang E.F., Meeker M., Holland M.C. Acute traumatic intraparenchymal hemorrhage: risk factors for progression in the early post-injury period. Neurosurgery. 2006;58(4) doi: 10.1227/01.NEU.0000197101.68538.E6. [DOI] [PubMed] [Google Scholar]
- 17.Sheng J., Li T., Zhuang D., et al. The monocyte-to-lymphocyte ratio at hospital admission is a novel predictor for acute traumatic intraparenchymal hemorrhage expansion after cerebral contusion. Mediators Inflamm. 2020;2020 doi: 10.1155/2020/5483981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhuang D., Sheng J., Peng G., et al. Neutrophil to lymphocyte ratio predicts early growth of traumatic intracerebral haemorrhage. Ann Clin Transl Neurol. 2021;8(8):1601–1609. doi: 10.1002/acn3.51409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sheng J., Yang J., Cai S., et al. Development and external validation of a novel multihematoma fuzzy sign on computed tomography for predicting traumatic intraparenchymal hematoma expansion. Sci. Rep. 2021;11(1) doi: 10.1038/s41598-021-81685-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Allison R.Z., Nakagawa K., Hayashi M., Donovan D.J., Koenig M.A. Derivation of a predictive score for hemorrhagic progression of cerebral contusions in moderate and severe traumatic brain injury. Neurocrit Care. 2017;26(1) doi: 10.1007/s12028-016-0303-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fletcher-Sandersjoo A., Wettervik T.S., Tatter C., et al. Absolute contusion expansion is superior to relative expansion in predicting traumatic brain injury outcomes: a multi-center observational cohort study. J. Neurotrauma. 2024;41(5–6) doi: 10.1089/neu.2023.0274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Han M.H., Kim J.M., Yi H.J., et al. Predictors of supratentorial deep intracerebral hemorrhage volume and their effect on short-term mortality in asians. Cerebrovasc. Dis. 2016;42(5–6) doi: 10.1159/000446552. [DOI] [PubMed] [Google Scholar]
- 23.Wang J., Wang D., Bian L., et al. Subarachnoid extension and unfavorable outcomes in patients with supratentorial intracerebral hemorrhage. BMC Neurol. 2023;23(1) doi: 10.1186/s12883-023-03087-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo S., Yang W.S., Shen Y.Q., et al. The clinical value of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and D-dimer-to-fibrinogen ratio for predicting pneumonia and poor outcomes in patients with acute intracerebral hemorrhage. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.1037255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Chung K.J., Kuang H., Federico A., et al. Semi-automatic measurement of intracranial hemorrhage growth on non-contrast CT. Int. J. Stroke. 2021;16(2) doi: 10.1177/1747493019895704. [DOI] [PubMed] [Google Scholar]
- 26.Li J., Shen D., Zhou Y., et al. Underlying microangiopathy and functional outcome of simultaneous multiple intracerebral hemorrhage. Front. Aging Neurosci. 2022;14 doi: 10.3389/fnagi.2022.1000573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sheng J., Chen W., Zhuang D., et al. A clinical predictive nomogram for traumatic brain parenchyma hematoma progression. Neurol Ther. 2022;11(1) doi: 10.1007/s40120-021-00306-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Adatia K., Newcombe V.F.J., Menon D.K. Contusion progression following traumatic brain injury: a review of clinical and radiological predictors, and influence on outcome. Neurocrit Care. 2021;34(1) doi: 10.1007/s12028-020-00994-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yuan F., Ding J., Chen H., et al. Predicting progressive hemorrhagic injury after traumatic brain injury: derivation and validation of a risk score based on admission characteristics. J. Neurotrauma. 2012;29(12) doi: 10.1089/neu.2011.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu W., Fan M., Lu W., Zhu W., Meng L., Lu S. Emerging roles of T helper cells in non-infectious neuroinflammation: savior or sinner. Front. Immunol. 2022;13 doi: 10.3389/fimmu.2022.872167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Z.H., Chen N.Y., Tu P.H., et al. Dha attenuates cerebral edema following traumatic brain injury via the reduction in blood–brain barrier permeability. Int. J. Mol. Sci. 2020;21(17) doi: 10.3390/ijms21176291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sîrbulescu R.F., Chung J.Y., Edmiston W.J., Poznansky S.A., Poznansky M.C., Whalen M.J. Intraparenchymal application of mature B lymphocytes improves structural and functional outcome after contusion traumatic brain injury. J. Neurotrauma. 2019;36(17) doi: 10.1089/neu.2018.6368. [DOI] [PubMed] [Google Scholar]
- 33.Simard J.M., Kilbourne M., Tsymbalyuk O., et al. Key role of sulfonylurea receptor 1 in progressive secondary hemorrhage after brain contusion. J. Neurotrauma. 2009;26(12) doi: 10.1089/neu.2009.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martínez-Valverde T., Vidal-Jorge M., Martínez-Saez E., et al. Sulfonylurea receptor 1 in humans with post-traumatic brain contusions. J. Neurotrauma. 2015;32(19) doi: 10.1089/neu.2014.3706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maegele M., Schöchl H., Menovsky T., et al. Traumatic Brain Injury 2 Coagulopathy and Haemorrhagic Progression in Traumatic Brain Injury: Advances in Mechanisms, Diagnosis, and Management. 2017;16 doi: 10.1016/S1474-4422(17)30197-7. www.thelancet.com/neurology [DOI] [PubMed] [Google Scholar]
- 36.Rahaman P., Del Bigio M.R. Histology of brain trauma and hypoxia-ischemia. Acad Forensic Pathol. 2018;8(3) doi: 10.1177/1925362118797728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peng J., Luo T., Li X., et al. Imaging predictors of hemorrhagic progression of a contusion after traumatic brain injury: a systematic review and meta-analysis. Sci. Rep. 2024;14(1) doi: 10.1038/s41598-024-56232-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang K., Zhao D qing, Zhang J jun, et al. Risk factors of progressive brain contusion and relationship with outcome. Zhejiang Da Xue Xue Bao Yi Xue Ban. 2015;44(4) doi: 10.3785/j.issn.1008-9292.2015.07.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vedantam A., Yamal J.M., Rubin M.L., Robertson C.S., Gopinath S.P. Progressive hemorrhagic injury after severe traumatic brain injury: effect of hemoglobin transfusion thresholds. J. Neurosurg. 2016;125(5) doi: 10.3171/2015.11.JNS151515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanus G.Z., Tanriverdi T., Alver I., Aydin S., Uzan M. Evolving traumatic brain lesions: predictors and results of ninety-eight head-injured patients. Neurosurg. Q. 2004;14(2) doi: 10.1097/01.wnq.0000127718.06183.12. [DOI] [Google Scholar]
- 41.Siracuse J.J., Robich M.P., Gautam S., Kasper E.M., Moorman D.W., Hauser C.J. Antiplatelet agents, warfarin, and epidemic intracranial hemorrhage. Surgery. 2010;148(4) doi: 10.1016/j.surg.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Baldon I.V., Amorim A.C., Santana Larissa Marques, et al. The extravasation of contrast as a predictor of cerebral hemorrhagic contusion expansion, poor neurological outcome and mortality after traumatic brain injury: a systematic review and meta-analysis. PLoS One. 2020;15(7) doi: 10.1371/journal.pone.0235561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Orito K., Hirohata M., Nakamura Y., et al. Predictive value of leakage signs for pure brain contusional hematoma expansion. J. Neurotrauma. 2018;35(5) doi: 10.1089/neu.2017.5247. [DOI] [PubMed] [Google Scholar]
- 44.Huang A.P.H., Lee C.W., Hsieh H.J., et al. Early parenchymal contrast extravasation predicts subsequent hemorrhage progression, Clinical deterioration, and need for surgery in patients with traumatic cerebral contusion. J. Trauma Inj. Infect. Crit. Care. 2011;71(6) doi: 10.1097/TA.0b013e31822c8865. [DOI] [PubMed] [Google Scholar]
- 45.Newcombe V.F.J., Williams G.B., Outtrim J.G., et al. Microstructural basis of contusion expansion in traumatic brain injury: insights from diffusion tensor imaging. J. Cerebr. Blood Flow Metabol. 2013;33(6) doi: 10.1038/jcbfm.2013.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.He H., Liu J., Li C., et al. Predicting hematoma expansion and prognosis in cerebral contusions: a radiomics-clinical approach. J. Neurotrauma. 2024 doi: 10.1089/neu.2023.0410. Published online. [DOI] [PubMed] [Google Scholar]
- 47.Yang Q., Sun J., Guo Y., et al. Radiomics features on computed tomography combined with clinical-radiological factors predicting progressive hemorrhage of cerebral contusion. Front. Neurol. 2022;13 doi: 10.3389/fneur.2022.839784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang L., Zhuang Q., Wu G., et al. Combined radiomics model for prediction of hematoma progression and clinical outcome of cerebral contusions in traumatic brain injury. Neurocrit Care. 2022;36(2) doi: 10.1007/s12028-021-01320-2. [DOI] [PubMed] [Google Scholar]
- 49.Kang D., Park J.E., Kim Y.H., et al. Diffusion radiomics as a diagnostic modal for atypical manifestation of primary central nervous system lymphoma: development and multicenter external validation. Neuro Oncol. 2018;20(9) doi: 10.1093/neuonc/noy021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li Y., Liu X., Qian Z., et al. Genotype prediction of ATRX mutation in lower-grade gliomas using an MRI radiomics signature. Eur. Radiol. 2018;28(7) doi: 10.1007/s00330-017-5267-0. [DOI] [PubMed] [Google Scholar]
- 51.Luo C., Yang J., Liu Z., Jing D. Predicting the recurrence and overall survival of patients with glioma based on histopathological images using deep learning. Front. Neurol. 2023;14 doi: 10.3389/fneur.2023.1100933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Prasanna P., Patel J., Partovi S., Madabhushi A., Tiwari P. Radiomic features from the peritumoral brain parenchyma on treatment-naïve multi-parametric MR imaging predict long versus short-term survival in glioblastoma multiforme: preliminary findings. Eur. Radiol. 2017;27(10) doi: 10.1007/s00330-016-4637-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang D., Chen J., Xue Q., et al. Heterogeneity signs on noncontrast computed tomography predict hematoma expansion after intracerebral hemorrhage: a meta-analysis. BioMed Res. Int. 2018;2018 doi: 10.1155/2018/6038193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu J., Xu H., Chen Q., et al. Prediction of hematoma expansion in spontaneous intracerebral hemorrhage using support vector machine. EBioMedicine. 2019;43 doi: 10.1016/j.ebiom.2019.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lustenberger T., Kern M., Relja B., Wutzler S., Störmann P., Marzi I. The effect of brain injury on the inflammatory response following severe trauma. Immunobiology. 2016;221(3):427–431. doi: 10.1016/j.imbio.2015.11.011. [DOI] [PubMed] [Google Scholar]
- 56.Liesz A., Hu X., Kleinschnitz C., Offner H. Functional role of regulatory lymphocytes in stroke. Stroke. 2015;46(5) doi: 10.1161/STROKEAHA.114.008608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liesz A., Suri-Payer E., Veltkamp C., et al. Regulatory T cells are key cerebroprotective immunomodulators in acute experimental stroke. Nat Med. 2009;15(2) doi: 10.1038/nm.1927. [DOI] [PubMed] [Google Scholar]
- 58.Schwartz M., Moalem G. Beneficial immune activity after CNS injury: prospects for vaccination. J. Neuroimmunol. 2001;113(2) doi: 10.1016/S0165-5728(00)00447-1. [DOI] [PubMed] [Google Scholar]
- 59.Jennett B., Bond M. Assessment of outcome after severe brain damage. A practical Scale. Lancet. 1975;305(7905) doi: 10.1016/S0140-6736(75)92830-5. [DOI] [PubMed] [Google Scholar]
- 60.Marshall L.F., Marshall S.B., Klauber M.R., et al. The diagnosis of head injury requires a classification based on computed axial tomography. J. Neurotrauma. 1992;9(SUPPL. 1) [PubMed] [Google Scholar]
- 61.Van Den Berghe G., Schoonheydt K., Becx P., Bruyninckx F., Wouters P.J. Insulin therapy protects the central and peripheral nervous system of intensive care patients. Neurology. 2005;64(8) doi: 10.1212/01.WNL.0000158442.08857.FC. [DOI] [PubMed] [Google Scholar]
- 62.Laird A.M., Miller P.R., Kilgo P.D., Meredith J.W., Chang M.C. Relationship of early hyperglycemia to mortality in trauma patients. J. Trauma Inj. Infect. Crit. Care. 2004;56(5) doi: 10.1097/01.TA.0000123267.39011.9F. [DOI] [PubMed] [Google Scholar]
- 63.Chen W., Yang J., Li B., et al. Neutrophil to lymphocyte ratio as a novel predictor of outcome in patients with severe traumatic brain injury. J. Head Trauma Rehabil. 2018;33(1):E53–E59. doi: 10.1097/HTR.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 64.Zhu Y., Xu L., Lin S., Chen Y., Han P., Lu Z. Establishment and validation of a prediction model for intraparenchymal hematoma expansion in patients with cerebral contusion: a reliable Nomogram. Clin. Neurol. Neurosurg. 2022;212 doi: 10.1016/j.clineuro.2021.107079. [DOI] [PubMed] [Google Scholar]
- 65.Adediran T., Drumheller B.C., McCunn M., Stein D.M., Albrecht J.S. Sex differences in in-hospital complications among older adults after traumatic brain injury. J. Surg. Res. 2019;243 doi: 10.1016/j.jss.2019.05.053. [DOI] [PubMed] [Google Scholar]
- 66.Dreier J.P. The role of spreading depression, spreading depolarization and spreading ischemia in neurological disease. Nat Med. 2011;17(4) doi: 10.1038/nm.2333. [DOI] [PubMed] [Google Scholar]
- 67.Tong W.S., Zheng P., Zeng J.S., et al. Prognosis analysis and risk factors related to progressive intracranial haemorrhage in patients with acute traumatic brain injury. Brain Inj. 2012;26(9) doi: 10.3109/02699052.2012.666437. [DOI] [PubMed] [Google Scholar]
- 68.Fischer P., Tamim I., Sugimoto K., et al. Spreading depolarizations suppress hematoma growth in hyperacute intracerebral hemorrhage in mice. Stroke. 2023;54(10) doi: 10.1161/STROKEAHA.123.042632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hartings J.A., Andaluz N., Bullock M.R., et al. Prognostic value of spreading depolarizations in patients with severe traumatic brain injury. JAMA Neurol. 2020;77(4) doi: 10.1001/jamaneurol.2019.4476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hartings J.A., Bullock M.R., Okonkwo D.O., et al. Spreading depolarisations and outcome after traumatic brain injury: a prospective observational study. Lancet Neurol. 2011;10(12) doi: 10.1016/S1474-4422(11)70243-5. [DOI] [PubMed] [Google Scholar]
- 71.Dreier J.P., Major S., Manning A., et al. Cortical spreading ischaemia is a novel process involved in ischaemic damage in patients with aneurysmal subarachnoid haemorrhage. Brain. 2009;132(7) doi: 10.1093/brain/awp102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ayata C., Lauritzen M. Spreading depression, spreading depolarizations, and the cerebral vasculature. Physiol. Rev. 2015;95(3) doi: 10.1152/physrev.00027.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fischer P., Sugimoto K., Chung D.Y., et al. Rapid hematoma growth triggers spreading depolarizations in experimental intracortical hemorrhage. J Cerebr Blood F Met. 2021;41(6):1264–1276. doi: 10.1177/0271678X209519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kazui S., Naritomi H., Yamamoto H., Sawada T., Yamaguchi T. Enlargement of spontaneous intracerebral hemorrhage: incidence and time course. Stroke. 1996;27(10) doi: 10.1161/01.STR.27.10.1783. [DOI] [PubMed] [Google Scholar]
- 75.Mayer S.A. Ultra-early hemostatic therapy for intracerebral hemorrhage. Stroke. 2003;34(1) doi: 10.1161/01.STR.0000046458.67968.E4. [DOI] [PubMed] [Google Scholar]
- 76.Brouwers H.B., Chang Y., Falcone G.J., et al. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71(2) doi: 10.1001/jamaneurol.2013.5433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Davis S.M., Broderick J., Hennerici M., et al. Hematoma growth is a determinant of mortality and poor outcome after intracerebral hemorrhage. Neurology. 2006;66(8) doi: 10.1212/01.wnl.0000208408.98482.99. [DOI] [PubMed] [Google Scholar]
- 78.Wang X., Arima H., Al-Shahi Salman R., et al. Clinical prediction algorithm (BRAIN) to determine risk of hematoma growth in acute intracerebral hemorrhage. Stroke. 2015;46(2) doi: 10.1161/STROKEAHA.114.006910. [DOI] [PubMed] [Google Scholar]