Abstract
Background
Numerous studies have been conducted to manage anemia in surgical patients through iron supplementation as an alternative to blood transfusion. However, patients with locally advanced rectal cancer have often been excluded from these studies, due to their standard treatment involving neoadjuvant chemoradiotherapy. This study aims to evaluate the impact of intravenous versus oral iron supplementation on iron deficiency anemia in patients with rectal cancer receiving preoperative chemoradiotherapy.
Methods
This open-label, single-center, parallel, superiority, randomized trial includes patients with primary rectal cancer who are candidates for preoperative chemoradiotherapy and have confirmed iron-deficiency anemia. A total of 94 patients will be randomly assigned in a 1:1 ratio to receive either intravenous or oral iron supplementation. Stratification factors include age (> 70 vs. ≤ 70 years) and baseline serum hemoglobin levels (7–10 g/dL vs. 10–13 g/dL). The primary endpoint is the percentage of patients achieving normalized hemoglobin levels from the start of treatment to the day of admission for surgery. Secondary endpoints include changes in serum hemoglobin from baseline to postoperatively, changes in iron assay parameters, time needed to hemoglobin normalization, volume of blood transfusions required, and incidence of postoperative complications.
Discussion
This study is the first randomized controlled trial investigating the effect of iron supplementation in iron-deficient patients with rectal cancer undergoing neoadjuvant chemoradiotherapy. This trial is expected to provide evidence for the benefits of administering iron supplementation in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy.
Trial registration
Clinical Research Information Service (CRIS) of Republic of Korea, KCT0009260, Registered on March 21, 2024.
Keywords: Rectal neoplasms, Iron-deficiency anemia, Chemoradiotherapy, Iron supplementation
Administrative information
Note: the numbers in curly brackets in this protocol refer to SPIRIT checklist item numbers. The order of the items has been modified to group similar items (see http://www.equator-network.org/reporting-guidelines/spirit-2013-statement-defining-standard-protocol-items-for-clinical-trials/).
| Title {1} | Intravenous versus oral iron supplementation for iron deficiency anemia in patients with rectal cancer undergoing neoadjuvant chemoradiotherapy: a study protocol for a randomized controlled trial |
| Trial registration {2a and 2b}. |
Clinical Research Information Service (CRIS) of Republic of Korea, KCT0009260, Registered on March 21, 2024. Our dataset appropriately fulfills the items from the World Health Organization Trial Registration Data Set. |
| Protocol version {3} | The current study protocol version is 1.5, which was approved on May 29, 2024. |
| Funding {4} | This work was supported by a grant (HCRI24017) from the Chonnam National University Hwasun Hospital, Institute of Biomedical Science. |
| Author details {5a} |
Hyeung-min Park1, Jaram Lee1, Soo Young Lee1, Chang Hyun Kim1, and Hyeong Rok Kim1 1Department of Surgery, Chonnam National University Hwasun Hospital and Medical School, Hwasun, Republic of Korea Soo Young Lee and Chang Hyun Kim equally contributed as co-corresponding authors. Correspondence to: Soo Young Lee (syleecrs@gmail.com), Chang Hyun Kim (cksantiago8@gmail.com) |
| Name and contact information for the trial sponsor {5b} | Chonnam National University Hwasun Hospital, Institute of Biomedical Science. 80–1899-0000 |
| Role of sponsor {5c} | Sponsor and funders of this study did not contribute to the study’s design and will not be involved in its implementation, analysis, or decision to publish. |
Introduction
Background and rationale {6a}
The incidence of colorectal cancer (CRC) ranks third among all cancers, and CRC is the second most common cause of cancer-related mortality [1]. Rectal cancer accounts for approximately 38% of CRC cases [2], with 46% of rectal cancer patients reported to experience anemia [3]. Among these patients, iron deficiency anemia (IDA) is the most prevalent type, accounting for up to 80% of anemia cases in rectal cancer [4]. Preoperative anemia in surgical patients is an independent risk factor for increased postoperative complications, prolonged hospital stays, and higher rates of 30-day morbidity and mortality [5–8]. To mitigate these adverse effects and complications and to improve recovery, the Enhanced Recovery After Surgery Society guidelines emphasized correcting anemia before surgery as part of prehabilitation [9].
Three strategies are commonly performed to correct anemic states: erythropoietin therapy, blood transfusion, and iron supplementation. However, the use of erythropoietin in cancer patients has been associated with increased risks of deep vein thrombosis or pulmonary embolism [10], and a 17% increase in overall mortality in cancer patients has been reported regarding erythropoietin-stimulating agents [6]. Additionally, several meta-analyses on perioperative transfusions in cancer surgery have found an increased risk of post-operative infection and surgical re-intervention as well as decreased overall and cancer-specific survival [5, 11]. Current guidelines for preoperative and postoperative care generally recommend minimizing blood transfusions [9]. For these reasons, iron supplementation is likely the most desirable and appropriate method for patients with preoperative anemia.
Two methods of iron supplementation are available: oral medication and intravenous infusion. While oral iron administration is considerably less expensive than intravenous supplementation, it is often associated with gastrointestinal side effects such as constipation, diarrhea, or abdominal pain [12]. Conversely, intravenous iron supplementation is generally well tolerated, though it carries risks of allergic and infusion reactions [10]. A randomized controlled trial comparing oral versus intravenous iron supplementation found that intravenous iron supplementation was associated with fewer anemic patients at the time of surgery and better quality of life scores [13, 14].
Previous studies have explored the correction of preoperative anemia through iron supplementation in patients who were not undergoing neoadjuvant treatment [14–16]. In these studies, elective surgeries were performed without delay, occurring 2 to 3 weeks after intravenous iron supplementation. Based on the results of these studies, a longer duration was suggested to achieve adequate increases in hemoglobin levels [17]. In this context, for patients with locally advanced rectal cancer who were excluded from previous research, neoadjuvant chemoradiotherapy (CRT) is routinely performed, requiring approximately 3 months from CRT to surgery. Talboom et al. [16] showed that it took 2 to 3 months for over 75% of patients to achieve anemia correction after receiving intravenous iron administration.
Objectives {7}
Our study focused on the correction of preoperative IDA in patients with locally advanced rectal cancer. For these patients, preoperative CRT followed by surgery is the standard treatment, allowing sufficient time to correct IDA before surgery. Therefore, we designed this randomized controlled trial to identify the effect of intravenous and oral iron supplementation for IDA in patients with rectal cancer undergoing neoadjuvant CRT.
The primary objective of this study is to evaluate the percentage of patients who have recovered from IDA on the day of admission for surgery following iron supplementation in conjunction with CRT. As secondary objectives, we will assess serum hemoglobin levels, iron assay parameters (serum iron, ferritin, total iron-binding capacity [TIBC]), length of hospital stay, time interval to achieve normalized serum hemoglobin, the need for and volume of blood transfusions, and perioperative complications.
Trial design {8}
This is an open-label, single-center, parallel, superiority, randomized, phase IV trial with a 1:1 allocation ratio to either the intravenous iron infusion or oral iron administration. The study flow of the assessment, intervention, and follow-up is shown in Fig. 1.
Fig. 1.
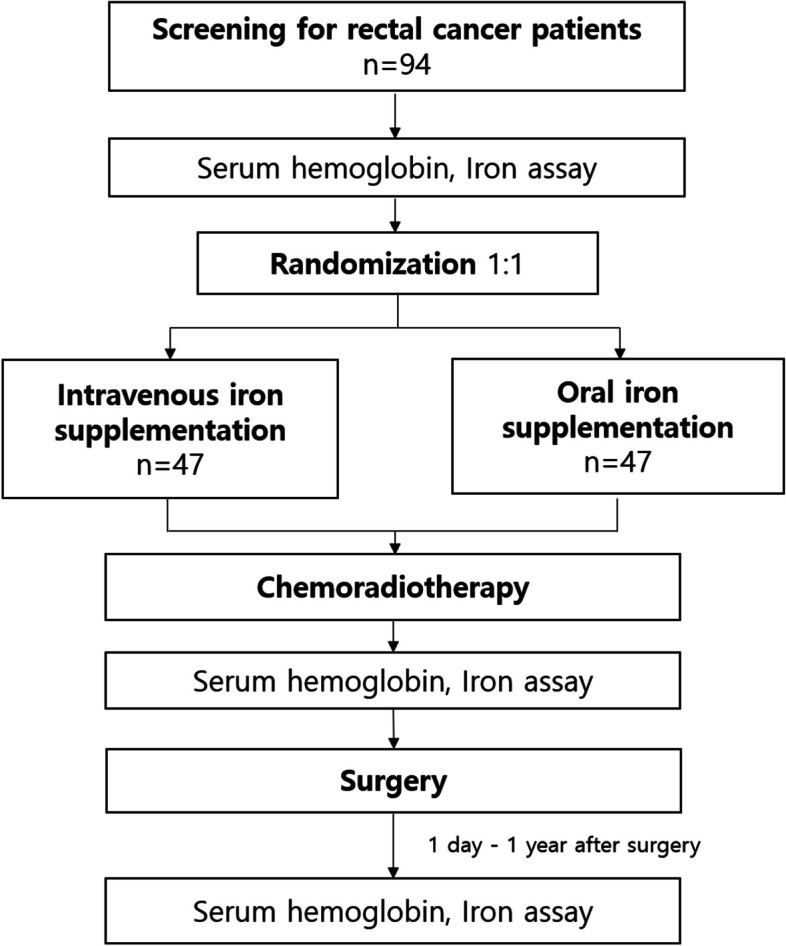
Flow chart
Methods: participants, interventions and outcomes
Study setting {9}
Patients eligible for this clinical trial will be enrolled at the Chonnam National University Hwasun Hospital in the Republic of Korea.
Eligibility criteria {10}
Patients with primary rectal cancer without distant metastasis are eligible if they meet the following inclusion criteria: over 20 years of age, agree to participate in the study and sign the clinical trial consent form; have IDA (serum hemoglobin < 12 g/dL for women or < 13 g/dL for men, serum ferritin < 30 ng/mL, serum transferrin saturation < 20%), and are scheduled to receive neoadjuvant CRT. Distant metastasis is defined as the presence of cancerous lesions beyond the primary tumor site, identified in other organs or the peritoneum through imaging studies.
Patients meeting at least one of the following criteria are ineligible from this trial: cases requiring emergency surgery (e.g., obstruction, or perforation), received a blood transfusion within 1 month of screening, serum ferritin > 800 ng/mL, women who are pregnant or non-compliance to contraceptive methods, cases with cancers other than rectal cancer, stage IV rectal cancer patients who are not candidates for neoadjuvant CRT, patients receiving treatments other than neoadjuvant CRT, cases contraindicated for the use of Ferinject Injection or Feroba-you tablet, the American Society of Anesthesiologists score > 3, use of erythropoietin-stimulating agents within 3 months of screening, chronic renal disease (glomerular filtration rate < 30 mL/min/m2), serum aspartate aminotransferase (glutamic oxalacetic transaminase) and alanine aminotransferase (glutamic pyruvic transaminase) levels more than 3 times the normal range, myelodysplastic syndrome, hereditary hemochromatosis, thalassemia, hemolytic anemia, history of abdominal surgery (excluding appendectomy, cholecystectomy, benign gynecological surgeries such as oophorectomy, cesarean section, and simple hysterectomy for fibroids).
Who will take informed consent? {26a}
The surgeon responsible for each enrolled patient will explain this trial at their outpatient clinic and obtain informed consent.
Additional consent provisions for collection and use of participant data and biological specimens {26b}
N/A. There will be no additional data collection for ancillary studies.
Interventions
Explanation for the choice of comparators {6b}
Feroba-you tablet is a dried ferrous sulfate that is widely used in actual clinical practice for oral iron supplementation, and its safety is tolerable, so it was designated as a comparator.
Intervention description {11a}
Iron supplementation will be initiated on the day of randomization. Patients assigned to the intravenous iron supplementation group will receive ferric carboxymaltose in doses based on body weight and serum hemoglobin levels (Table 1). Patients with serum hemoglobin ≤ 10 g/dL (6.2 mmol/L) will receive a dose of 1500 mg if their weight is 35 to 70 kg or 2000 mg if over 70 kg. Patients with serum hemoglobin > 10 g/dL (6.2 mmol/L) will receive a dose of 1000 mg if their weight is 35 to 70 kg or 1500 mg if over 70 kg. The dose per administration should not exceed 1000 mg, and administrations should not occur more than once per week. Therefore, if an additional infusion for the remainder of the dose is required, it will be administered 1 week after the initial infusion. Patients assigned to the oral iron supplementation group will take 256 mg of ferrous sulfate, two tablets a day (one in the morning and one in the evening), for a total daily dose of 512 mg, until the day before surgery. If anemia persists after surgery, they will continue taking the oral medication until the anemia is corrected. Ferric carboxymaltose will be administered intravenously by clinical staff in a designated injection room, following protocol guidelines, while ferrous sulfate will be self-administered orally by patients based on the dosage instructions provided by the study team.
Table 1.
Cumulative dose of ferric carboxymaltose based on body weight and serum hemoglobin
| Serum hemoglobin (g/dL) | Patient weight: 35 to 70 kg | Patient weight: > 70 kg |
|---|---|---|
| < 10 | 1500 mg | 2000 mg |
| ≥ 10 | 1000 mg | 1500 mg |
Criteria for discontinuing or modifying allocated interventions {11b}
Participants are free to discontinue their participation at any time, for any reason, without any consequences. If a participant meets previously unrecognized exclusion criteria or develops an urgent medical condition that disqualifies participating, the investigator may choose to discontinue their participation in this study. If a patient discontinues from the trial, all data collected up to the point of discontinuation will be retained and included in the final analysis unless the patient explicitly requests the deletion of their data. No modifications to the intervention are planned for this trial. Any unforeseen changes will be documented and considered in the data analysis.
Strategies to improve adherence to interventions {11c}
Patients taking oral iron supplementation will receive a medication diary to improve adherence and maintain their medications as planned. The daily intake of oral iron recorded by participants will be reviewed to measure adherence.
Relevant concomitant care permitted or prohibited during the trial {11d}
Blood transfusions will be permitted based on international guidelines [18]. Transfusions will be administered when serum hemoglobin is below 7 g/dL for hematologically stable adult patients and below 8 g/dL for patients with cardiovascular disease.
Provisions for post-trial care {30}
In case of any adverse events related to the research procedures, appropriate medical treatment will be provided, and compensation will be given based on the established regulations of our institution.
Outcomes {12}
Our primary endpoint is the percentage of patients achieving normalization of serum hemoglobin levels on the day of admission for surgery (serum hemoglobin ≥ 12 g/dL for women and ≥ 13 g/dL for men). Our secondary endpoints include serum hemoglobin levels, iron assay parameters (serum iron, ferritin, TIBC), length of hospital stay, time interval to achieve normalized serum hemoglobin levels, the necessity and volume of blood transfusions, and perioperative complications (Clavien-Dindo classification, Comprehensive Complication Index) [19, 20]. Blood samples to assess hemoglobin will be taken at baseline, after CRT, at admission, and on postoperative day 1, as well as at 1 week, 4 weeks, 8 weeks, 3 months, 6 months, and 1 year, to compare the effects of intravenous and oral iron supplementation at each time point. Iron values will be measured after CRT, on postoperative day 1, at 1 week, and at 4 weeks. All iron assay parameters in this study can be assessed through serum laboratory tests. Iron deficiency will be determined by serum ferritin levels of less than 30 ng/mL and serum transferrin saturation (serum iron × 100/TIBC) of less than 20%. The length of hospital stay will be measured from the day of admission for rectal cancer surgery until the day of discharge following the surgery. The time interval to achieve normalized serum hemoglobin levels will be compared, with a focus on the values identified after CRT. The necessity for blood transfusion will be determined by international guidelines [18], with the transfusion volume adjusted based on individual patient factors, including underlying disease and intraoperative bleeding.
Participant timeline {13}
At the initial outpatient visit, an assessment will be conducted to diagnose IDA and confirm eligibility based on the inclusion criteria, followed by obtaining informed consent. Eligible patients will undergo randomization at the next outpatient visit, and iron supplementation will be initiated. Ferinject injection as intravenous iron supplementation will be administered either once or twice at weekly intervals, while ferrous sulfate as oral iron supplementation will be continued until just before surgery. Follow-up will maintain until 1-year post-operation, with scheduled hematologic tests (complete blood count, albumin, C-reactive protein) and iron assays (serum iron, ferritin, TIBC) performed. The detailed schedule of assessments is presented in Table 2.
Table 2.
Schedule of assessments
| Study period | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Enrolment | Allocation | Post-allocation | Close-out | ||||||||
| Timepoint | t1 | t2 |
t3 (after CRT) |
t4 (adm for op) |
t5 (po at 1 day) |
t6 (po at 1 wk) |
t7 (po at 4 wk) |
t8 (po at 8 wk) |
t9 (po at 3 mo) |
t10 (po at 6 mo) |
t11 (po at 1 year) |
| Enrolment: | |||||||||||
| Eligibility screen | X | ||||||||||
| Informed consent | X | ||||||||||
| Allocation | X | ||||||||||
| Interventions: | |||||||||||
| [Ferinject] | X | ||||||||||
| [Ferrous sulfate] | |||||||||||
| Assessments: | |||||||||||
| [Serum hemoglobin] | X | X | X | X | X | X | X | X | X | X | |
| [Iron assay] | X | X | X | X | X | ||||||
CRT chemoradiotherapy, adm admission, op operation, po post-operation, wk week, mo month
Sample size {14}
Based on a previous randomized controlled trial [16], we hypothesized that the rate of achieving normalization of serum hemoglobin levels 3 months after initiating iron supplementation would be 70% in the experimental arm and 40% in the control arm. With a two-sided α of 0.05 and a power of 0.8, the sample size was calculated as 94 patients (47 per group), considering an estimated drop-out rate of 10%.
Recruitment {15}
All rectal cancer patients visiting the outpatient clinic will be closely monitored to determine if they meet the inclusion criteria. Once eligibility is confirmed, the surgeon will contact the patient to provide details about the study background and process and request participation in the study. Patients will be given ample time to consider participation and to ask any questions they may have.
Assignment of interventions: allocation
Sequence generation {16a}
Random assignment will be performed in a 1:1 ratio using computer-generated random numbers, stratified by age (> 70 vs. ≤ 70 years) and baseline serum hemoglobin levels (7–10 g/dL vs. 10–13 g/dL). The random sequences will be generated using permuted block randomization methods. Given a block size of four and two stratification variables (age and serum hemoglobin level), we will use a total of 48 blocks across all strata.
Concealment mechanism {16b}
N/A. In this trial, concealing the sequence is not necessary because the intervention will be performed immediately after the randomization results are obtained. Patients will be randomized during their second outpatient visit, receive their results on the same day within the hospital, and begin iron supplementation immediately afterward. The results will be provided in person by the researcher.
Implementation {16c}
Allocation sequences will be generated independently by a designated researcher not involved in the outcome data analysis. Participants will be enrolled by the surgeon at each outpatient clinic. The assignment of participants to interventions will be performed and overseen by the designated researcher, who will also be responsible for prescribing the intravenous iron infusions or oral iron medications.
Assignment of interventions: blinding
Who will be blinded {17a}
All surgeons and other investigators will be blinded, except for the researcher who will know the arm assignment and will prescribe intravenous iron infusions or oral iron medications. All study-related injectable and oral medications will be labeled with unique codes, and only the designated researcher will be able to identify the assigned groups. These codes will be maintained until the final analysis, ensuring blinding is preserved throughout the trial. Due to the nature of the study, the patients will not be blinded.
Procedure for unblinding if needed {17b}
N/A. Participants will not be blinded in this trial.
Data collection and management
Plans for assessment and collection of outcomes {18a}
To ensure consistent assessment, all investigators will receive uniform training annually. By performing laboratory testing at an accredited tertiary university hospital, we will ensure a high level of reliability and validity.
Plans to promote participant retention and complete follow-up {18b}
During follow-up, we will collect detailed contact preferences and send text messages prior to follow-up visits to maximize participant retention. Up to five contact attempts will be made before a participant is considered lost to follow-up.
Data management {19}
Outcome assessors will first enter data into registered paper-based case report forms and then into predesigned electronic case report forms. Because medical notes are not structured to store and organize the specific data required for the clinical trial, these case report forms will be prepared. In the electronic case report form, range checks for data values will be conducted to ensure all entered data fall within predefined acceptable limits, further enhancing data accuracy. Using two types of case report forms will facilitate reliable data verification. Additionally, regular backups of the electronic case report forms will be performed to prevent data loss. Record retention will begin immediately after the trial initiation. All records will be maintained for inspection for up to 3 years after the completion of the study report. After the 3-year retention period, the data will be securely discarded based on the study protocol.
Confidentiality {27}
Participants’ identities will be protected using a study identification number that cannot be linked to personal information. Paper-based case report forms will be stored securely in a restricted location and are accessible only to research team members. Data stored in electronic case report forms will be secured on a password-protected server with access restricted to authorized researchers.
Plans for collection, laboratory evaluation and storage of biological specimens for genetic or molecular analysis in this trial/future use {33}
N/A. We have no plan to collect biological specimens.
Statistical methods
Statistical methods for primary and secondary outcomes {20a}
Data analysis will be performed using intention-to-treat set and the per-protocol set. The intent-to-treat set includes all patients who were randomized, regardless of whether they receive each iron supplement. The per-protocol analysis set includes patients who treated according to the protocol, excluding those with major protocol violations. Considering that there will be a few patients expected to crossover, these analyses could be very closely matched. Categorical variables will be compared using the χ2 or Fisher’s exact test, and continuous variables will be compared using Student’s t-test or the Mann–Whitney U-test. All variables with a p-value < 0.05 will be considered significant. All statistical analyses will be performed using the R statistical software, version 3.4.3 (R Foundation for Statistical Computing).
Interim analyses {21b}
N/A. There will be no interim analysis because iron supplementation in this trial does not increase the risk for patients undergoing CRT and subsequent surgery.
Methods for additional analyses (e.g., subgroup analyses) {20b}
N/A. We have no plan for additional analyses.
Methods in analysis to handle protocol non-adherence and any statistical methods to handle missing data {20c}
Because the normalization of serum hemoglobin will be evaluated during hospitalization before surgery, we expect no missing data on the primary outcome. For other possible missing data, multiple imputation will be used under the assumption that the data are missing at random.
Plans to give access to the full protocol, participant-level data and statistical code {31c}
N/A. We have no plan to allow access to full protocol, participant-level dataset, and statistical code.
Oversight and monitoring
Composition of the coordinating center and trial steering committee {5d}
The trial steering committee (TSC) will be comprised of the surgeons participating in the study and will serve as the decision-making body responsible for the scientific conduct of the study. The TSC will meet every 3 weeks to ensure that the trial is conducted following relevant principles and to provide overall supervision. Day-to-day support will be provided by the principal investigator, who will oversee the trial, handle recruitment, and assume medical responsibility of the patients, and by the research coordinators, who will manage data collection and follow-up of the patients. The principal investigator and research coordinators will meet weekly.
Composition of the data monitoring committee, its role and reporting structure {21a}
The data monitoring committee will consist of researchers not involved with the study team. The entire data collection, recording, and management process will be monitored and validated by the committee, independently from the sponsor and any competing interests.
Adverse event reporting and harms {22}
Adverse events will be closely monitored during hospitalization for surgery. During the follow-up period, adverse events will be collected and assessed at each scheduled outpatient visit. Serious adverse events that could result in death or life-threatening situations will be reported by the investigators within 24 h of detection. Adverse event assessment in this trial will also include other serious adverse event criteria, such as prolonged hospitalization, significant disability or incapacity, and congenital malformations or birth defects. The principal investigator and research coordinators, who are responsible for providing day-to-day support, will assess the severity, outcomes, causality, and expectedness of the adverse events.
Frequency and plans for auditing trial conduct {23}
The institutional review board of Chonnam National University Hwasun Hospital will continue to review the trial, including consent forms, compliance with the protocol, planned interventions, and quality of data collected in the case report forms, at least annually. These audits will be independent of the investigators and the sponsor.
Plans for communicating important protocol amendments to relevant parties (e.g., trial participants, ethical committees) {25}
If any protocol revisions are identified as necessary during the trial, modifications may be made with the agreement of the investigator and the trial participants. The revised protocol will be discussed and approved by the institutional review board.
Dissemination plans {31a}
The results of this trial will be shared with participating researchers, patients, and the public through publication. The results will be published, regardless of the magnitude or direction of the effect.
Discussion
Previous randomized controlled trials comparing intravenous iron supplementation with control groups, which designated the primary outcome as the incidence and volume of blood transfusions, failed to demonstrate significant superiority [14, 15]. In the FIT trial [16], a significant difference in the normalization rate of hemoglobin between the experimental and control groups was observed 30 days after surgery (6 weeks after intravenous iron supplementation). In the PREVENT trial [15], a significant difference in mean hemoglobin levels between the two groups was identified 8 weeks after surgery (10 weeks after intravenous iron supplementation). These findings indicated that a sufficient interval would be necessary to achieve significant differences in the increase of hemoglobin.
Chemotherapy or radiation therapy has been known to impact bone marrow function, leading to myelosuppression and potentially causing anemia [21]. A retrospective analysis reported that the proportion of anemic patients with CRC increased from 53% before radiotherapy to 67% after radiotherapy. However, these reports may not accurately reflect the effects of current CRT practices. Furthermore, it has been reported that patients with anemia undergoing CRT for rectal cancer experience less tumor regression and poorer 3-year local control [22, 23]. Therefore, it would be reasonable to prioritize the treatment of anemia through iron supplementation while evaluating changes in hemoglobin levels related to CRT.
Anastomotic leakage is known to have a higher incidence in rectal surgery compared to colonic surgery [24, 25]. Because this study only includes patients with rectal cancer, the rate of surgical complications may be higher than in previous studies that also included patients with colon cancer [14, 16]. By correcting anemia, we will also evaluate the rate of surgical complications, including anastomotic leakage, to determine if there are significant differences.
In this trial, we aimed to evaluate the correction of anemia in rectal cancer patients undergoing intravenous and oral iron supplementation followed by CRT. Based on the results of this trial, we may establish the routine implementation of iron assays and the standardization of iron supplementation before preoperative CRT in patients with advanced rectal cancer. Given that overall blood donations have decreased by approximately 25% since COVID-19 [26], this approach could offer an alternative to address the shortage of blood supplies. Moreover, it could provide a basis for the treatment of anemia with iron supplementation in other organ cancers undergoing CRT.
Trial status
This trial is currently open for recruitment. The current study protocol version is 1.5, which was approved on May 29, 2024. The date recruitment began was May 14, 2024, and we anticipate reaching our maximum number of included patients by December 2026.
Acknowledgements
Not applicable.
Abbreviations
- CRC
Colorectal cancer
- IDA
Iron deficiency anemia
- CRT
Chemoradiotherapy
- TIBC
Total iron-binding capacity
- TSC
Trial steering committee
Authors’ contributions {31b}
SYL and CHK is the CI. They conceived the study, led the proposal, and developed the protocol. HMP contributed to the study design, protocol development, and manuscript writing. He also examined the statistical aspects of the protocol. JL and HRK provided advice on the study design and wrote the manuscript. All authors have read and approved the final version for publication. On any future trial publication, HMP and SYL will be the co-first authors. CHK and HRK will be the co-corresponding author. JL will be the third author.
Funding {4}
This work was supported by a grant (HCRI24017) from the Chonnam National University Hwasun Hospital, Institute of Biomedical Science. This funding source did not contribute to the study’s design and will not be involved in its implementation, analysis, or decision to publish.
Data availability {29}
Due to confidentiality and ethical restrictions related to patient privacy, we have no plans to share the data.
Declarations
Ethics approval and consent to participate {24}
The protocol of this study has been approved by the institutional review board of Chonnam National University Hwasun Hospital (IRB No. CNUHH-2024–030). The current version of the protocol is 1.5. All participants will be explained regarding the aims and process of the trial, possible results, and risks, as specified in the consent form, and will sign the informed consent form. Each participant will receive a copy of their signed consent form, which will be securely stored in a restricted location along with paper-based report forms. This investigation was registered on Clinical Research Information Service (CRIS registration No. KCT0009260; https://cris.nih.go.kr/cris/index/index.do).
Consent for publication {32}
All participants must provide written, informed consent for their participation as well as for the publication of their data in an aggregated and anonymized form. A consent form is available as Supplementary material.
Competing interests {28}
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Soo Young Lee and Chang Hyun Kim equally contributed as co-corresponding authors.
Contributor Information
Soo Young Lee, Email: syleecrs@gmail.com.
Chang Hyun Kim, Email: cksantiago8@gmail.com.
References
- 1.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–49. [DOI] [PubMed] [Google Scholar]
- 2.Hossain MS, Karuniawati H, Jairoun AA, Urbi Z, Ooi J, John A, et al. Colorectal cancer: a review of carcinogenesis, global epidemiology, current challenges, risk factors, preventive and treatment strategies. Cancers (Basel) 2022;14(7). [DOI] [PMC free article] [PubMed]
- 3.Gvirtzman R, Livovsky DM, Tahover E, Goldin E, Koslowsky B. Anemia can predict the prognosis of colorectal cancer in the pre-operative stage: a retrospective analysis. World J Surg Oncol. 2021;19(1):341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wilson MJ, Dekker JWT, Harlaar JJ, Jeekel J, Schipperus M, Zwaginga JJ. The role of preoperative iron deficiency in colorectal cancer patients: prevalence and treatment. Int J Colorectal Dis. 2017;32(11):1617–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256(2):235–44. [DOI] [PubMed] [Google Scholar]
- 6.Bohlius J, Schmidlin K, Brillant C, Schwarzer G, Trelle S, Seidenfeld J, et al. Recombinant human erythropoiesis-stimulating agents and mortality in patients with cancer: a meta-analysis of randomised trials. Lancet. 2009;373(9674):1532–42. [DOI] [PubMed] [Google Scholar]
- 7.Leichtle SW, Mouawad NJ, Lampman R, Singal B, Cleary RK. Does preoperative anemia adversely affect colon and rectal surgery outcomes? J Am Coll Surg. 2011;212(2):187–94. [DOI] [PubMed] [Google Scholar]
- 8.Musallam KM, Tamim HM, Richards T, Spahn DR, Rosendaal FR, Habbal A, et al. Preoperative anaemia and postoperative outcomes in non-cardiac surgery: a retrospective cohort study. Lancet. 2011;378(9800):1396–407. [DOI] [PubMed] [Google Scholar]
- 9.Gustafsson UO, Scott MJ, Hubner M, Nygren J, Demartines N, Francis N, et al. Guidelines for perioperative care in elective colorectal surgery: Enhanced Recovery After Surgery (ERAS(®)) society recommendations: 2018. World J Surg. 2019;43(3):659–95. [DOI] [PubMed] [Google Scholar]
- 10.Lynch KT, Hassinger TE. Preoperative identification and management of anemia in the colorectal surgery patient. Clin Colon Rectal Surg. 2023;36(3):161–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pang QY, An R, Liu HL. Perioperative transfusion and the prognosis of colorectal cancer surgery: a systematic review and meta-analysis. World J Surg Oncol. 2019;17(1):7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tolkien Z, Stecher L, Mander AP, Pereira DI, Powell JJ. Ferrous sulfate supplementation causes significant gastrointestinal side-effects in adults: a systematic review and meta-analysis. PLoS ONE. 2015;10(2): e0117383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keeler BD, Dickson EA, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, et al. The impact of pre-operative intravenous iron on quality of life after colorectal cancer surgery: outcomes from the intravenous iron in colorectal cancer-associated anaemia (IVICA) trial. Anaesthesia. 2019;74(6):714–25. [DOI] [PubMed] [Google Scholar]
- 14.Keeler BD, Simpson JA, Ng O, Padmanabhan H, Brookes MJ, Acheson AG. Randomized clinical trial of preoperative oral versus intravenous iron in anaemic patients with colorectal cancer. Br J Surg. 2017;104(3):214–21. [DOI] [PubMed] [Google Scholar]
- 15.Richards T, Baikady RR, Clevenger B, Butcher A, Abeysiri S, Chau M, et al. Preoperative intravenous iron to treat anaemia before major abdominal surgery (PREVENTT): a randomised, double-blind, controlled trial. Lancet. 2020;396(10259):1353–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Talboom K, Borstlap WAA, Roodbeen SX, Bruns ERJ, Buskens CJ, Hompes R, et al. Ferric carboxymaltose infusion versus oral iron supplementation for preoperative iron deficiency anaemia in patients with colorectal cancer (FIT): a multicentre, open-label, randomised, controlled trial. Lancet Haematol. 2023;10(4):e250–60. [DOI] [PubMed] [Google Scholar]
- 17.Hands K, Richards T. Iron therapy for preoperative anaemia. Lancet Haematol. 2023;10(4):e236–7. [DOI] [PubMed] [Google Scholar]
- 18.Carson JL, Stanworth SJ, Guyatt G, Valentine S, Dennis J, Bakhtary S, et al. Red blood cell transfusion: 2023 AABB International Guidelines. JAMA 2023. [DOI] [PubMed]
- 19.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Slankamenac K, Graf R, Barkun J, Puhan MA, Clavien PA. The comprehensive complication index: a novel continuous scale to measure surgical morbidity. Ann Surg. 2013;258(1):1–7. [DOI] [PubMed] [Google Scholar]
- 21.Schwartz RN. Anemia in patients with cancer: incidence, causes, impact, management, and use of treatment guidelines and protocols. Am J Health Syst Pharm 2007;64(3 Suppl 2):S5–13; quiz S28–30. [DOI] [PubMed]
- 22.Lee H, Park HC, Park W, Choi DH, Kim YI, Park YS, et al. Negative impact of pretreatment anemia on local control after neoadjuvant chemoradiotherapy and surgery for rectal cancer. Radiat Oncol J. 2012;30(3):117–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGrane JM, Humes DJ, Acheson AG, Minear F, Wheeler JMD, Walter CJ. Significance of anemia in outcomes after neoadjuvant chemoradiotherapy for locally advanced rectal cancer. Clin Colorectal Cancer. 2017;16(4):381–5. [DOI] [PubMed] [Google Scholar]
- 24.Kryzauskas M, Bausys A, Degutyte AE, Abeciunas V, Poskus E, Bausys R, et al. Risk factors for anastomotic leakage and its impact on long-term survival in left-sided colorectal cancer surgery. World J Surg Oncol. 2020;18(1):205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsalikidis C, Mitsala A, Mentonis VI, Romanidis K, Pappas-Gogos G, Tsaroucha AK, et al. Predictive factors for anastomotic leakage following colorectal cancer surgery: where are we and where are we going? Curr Oncol. 2023;30(3):3111–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kumar A, Kumari S, Saroj U, Verma A, Kiran KA, Prasad MK, et al. Impact of the COVID-19 pandemic on blood donation patterns: a systematic review and meta-analysis. Cureus. 2023;15(8): e43384. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Due to confidentiality and ethical restrictions related to patient privacy, we have no plans to share the data.


