Abstract
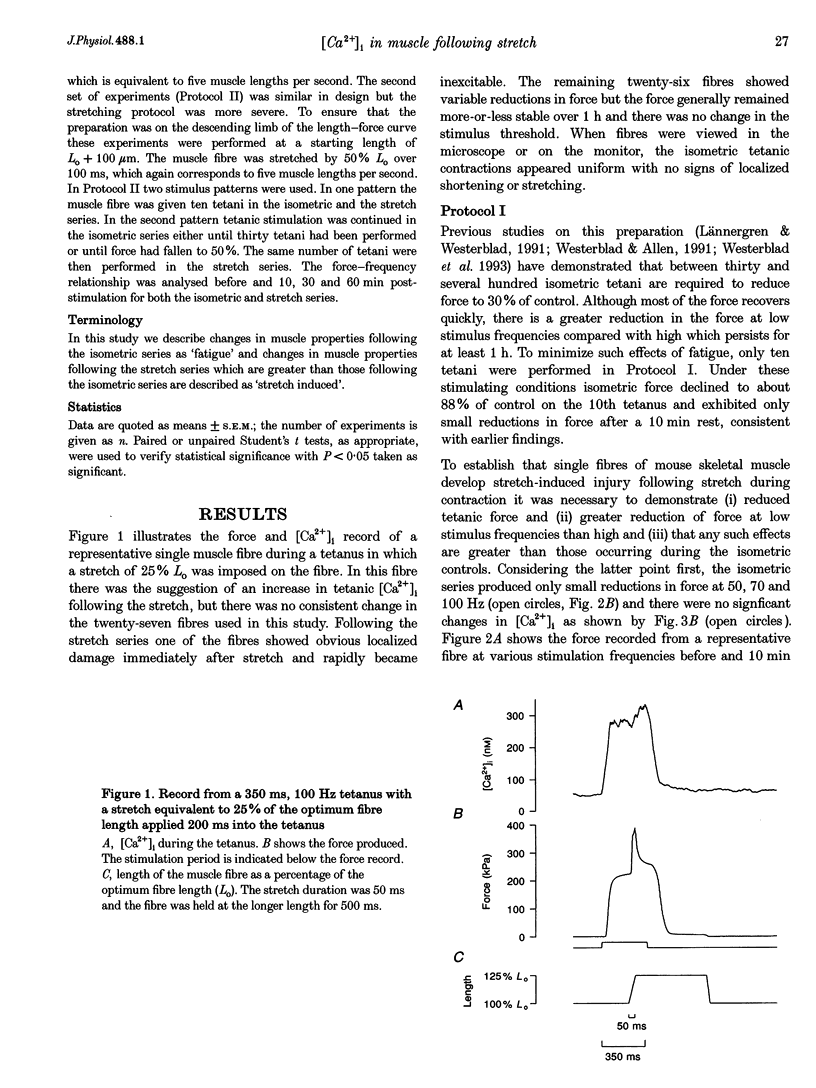
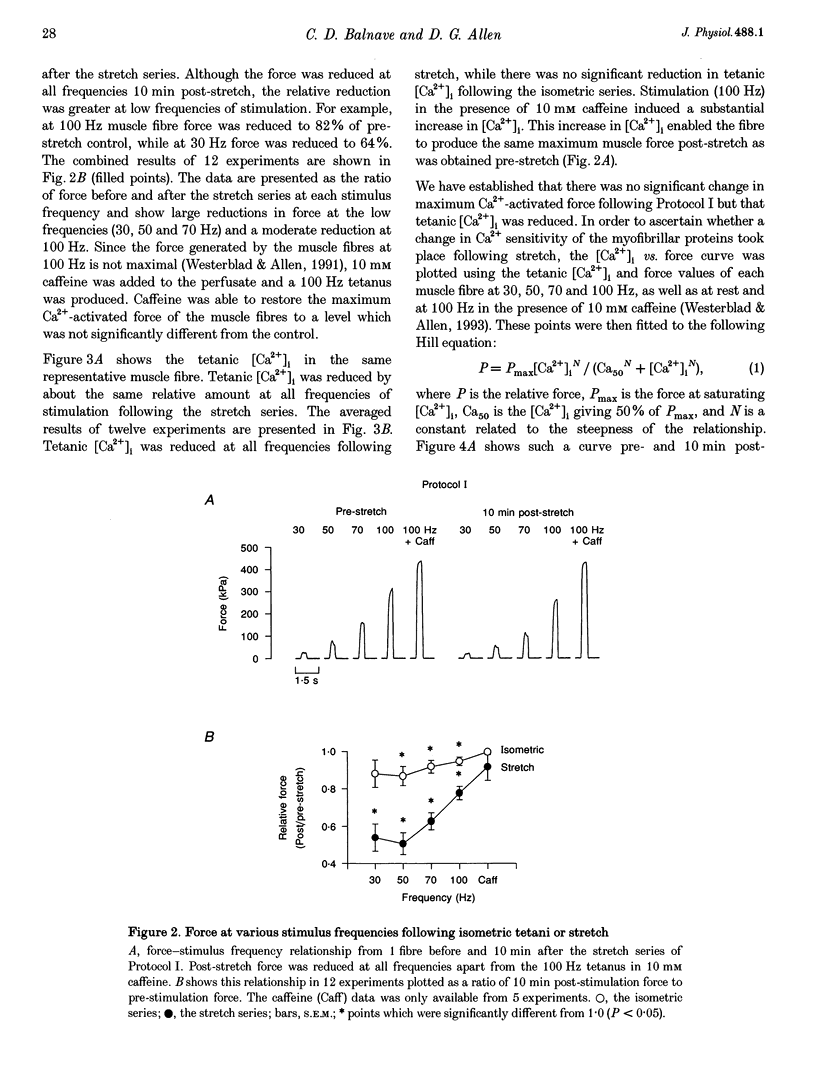
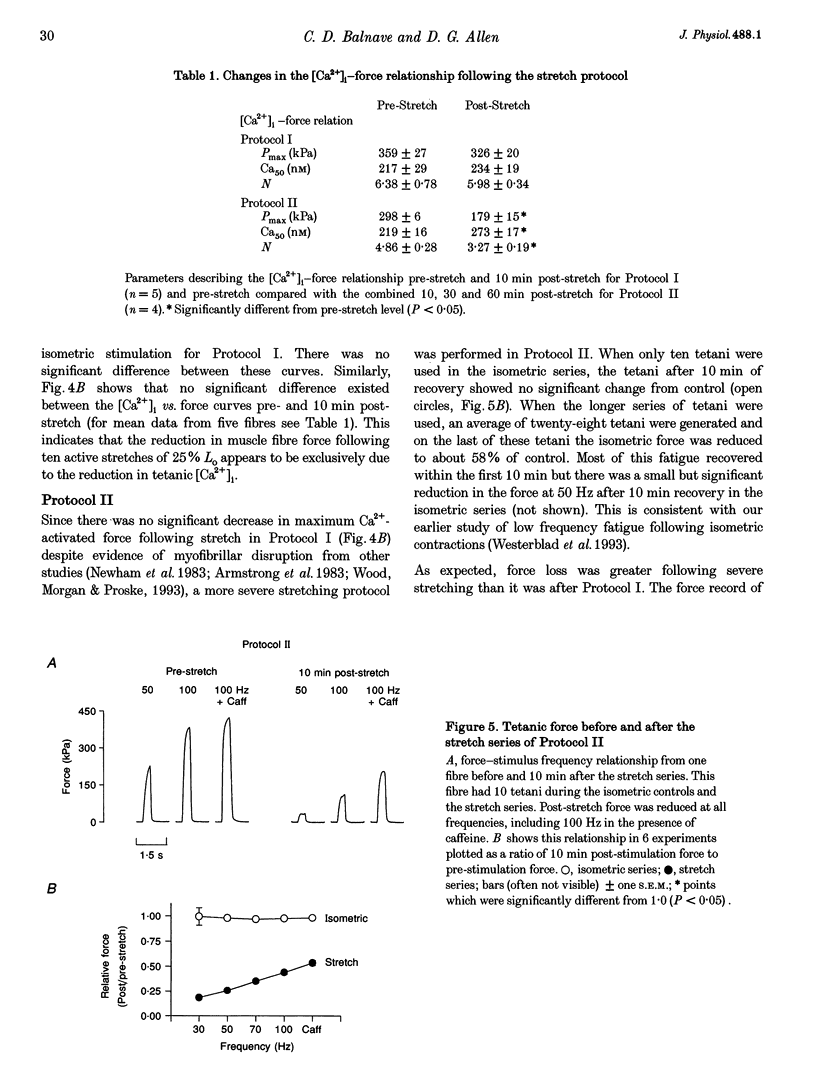
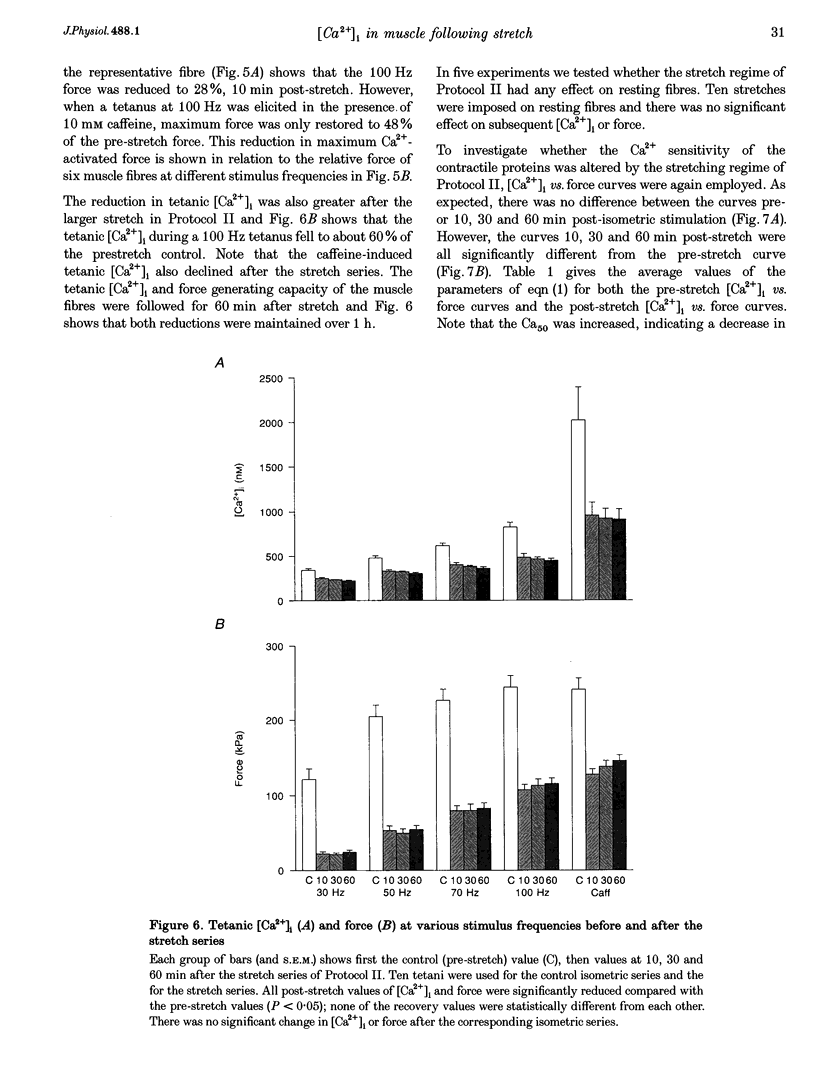
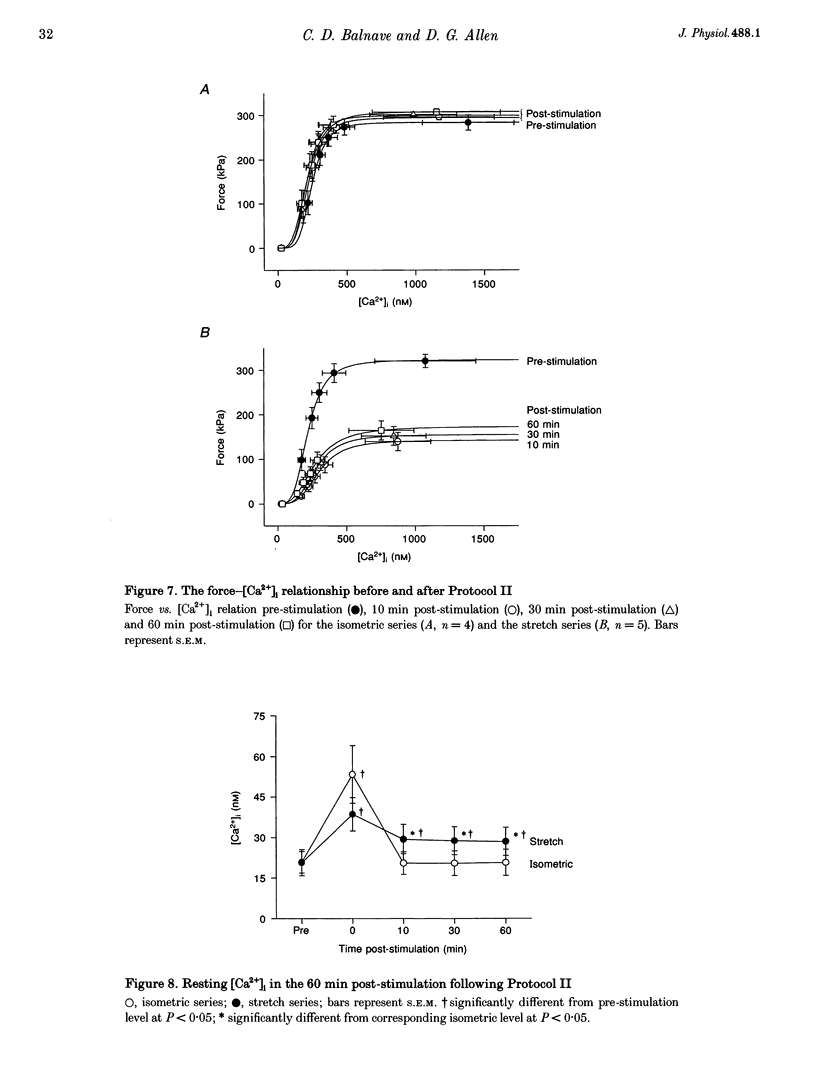
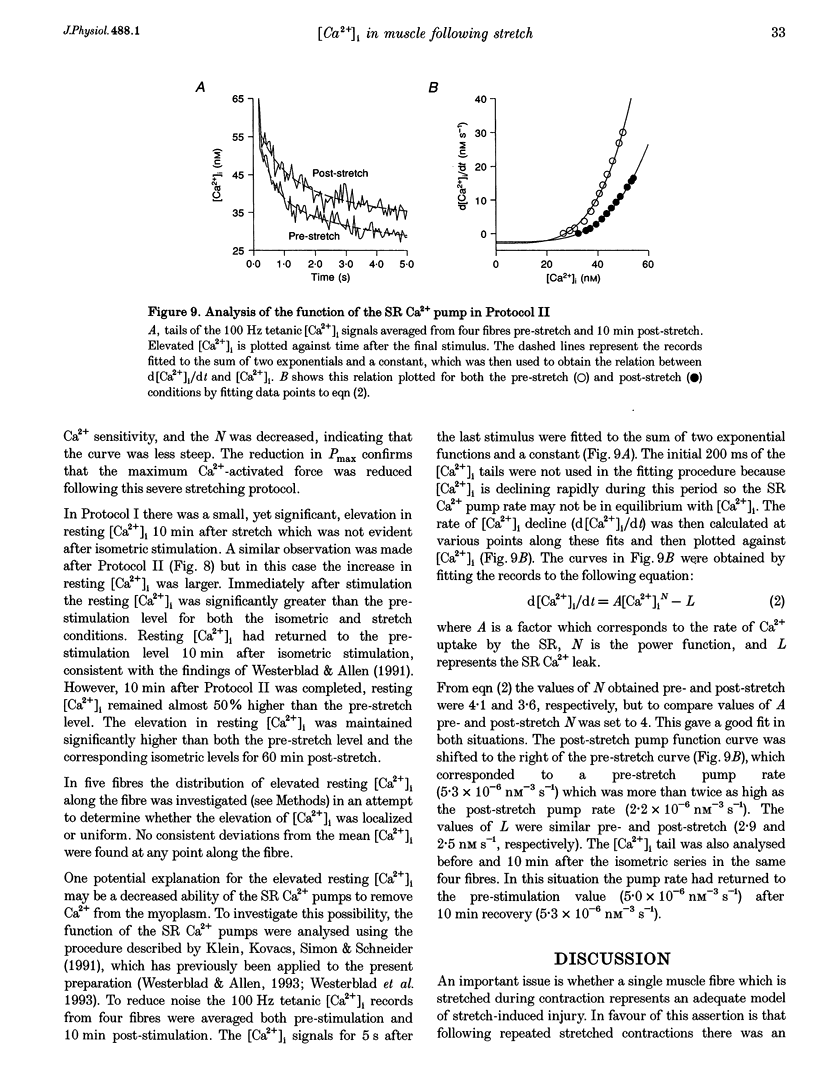
1. The role of the myoplasmic free Ca2+ concentration ([Ca2+]i) in the reduction of muscle force following contractions with stretch was investigated in single fibres from mouse toe muscle. Muscle fibres were either stretched by 25% of their optimum length (Lo) for ten tetani (Protocol I) or stretched by 50% of Lo for between ten and thirty tetani (Protocol II). Indo-1 was used to measure [Ca2+]i. 2. In each protocol the stretch series was compared with isometric controls; the stretch series always resulted in greater changes in muscle properties than in the isometric controls. The observed changes were (i) reduced tetanic force, (ii) reduced tetanic [Ca2+]i, (iii) increased resting [Ca2+]i and (iv) the greater relative reduction in force at low stimulus frequencies (30 and 50 Hz) compared with high (100 Hz). These changes were maintained for up to 60 min. 3. Stretching a resting muscle fibre had no effect on the subsequent [Ca2+]i or force. 4. Following Protocol I 10 mM caffeine restored tetanic force to pre-stretch levels. Tetanic [Ca2+]i vs. force curves were constructed pre- and post-stretch and showed that neither the maximum Ca(2+)-activated force nor the Ca2+ sensitivity of the muscle fibres post-stretch was significantly different from control. The force reduction, therefore, appears to be the result of reduced tetanic [Ca2+]i. 5. The more severe stretching regimen of Protocol II resulted in a much greater reduction in force than Protocol I. Ten millimolar caffeine did not restore control force. Comparison of the [Ca2+]i-force relationships pre- and post-stretch showed that the reduction in tetanic force was caused by a combination of a reduced tetanic [Ca2+]i, reduced maximum Ca(2+)-activated force and reduced Ca2+ sensitivity. 6. Following both protocols the resting [Ca2+]i showed a small rise which persisted for at least 60 min. This elevated [Ca2+]i was associated with a reduction in the pump rate of the sarcoplasmic reticulum Ca2+ pump. 7. This study establishes that reduced Ca2+ release and reduced Ca2+ sensitivity contribute to the reduction in force generating capacity of single mammalian muscle fibres following active stretches.
Full text
PDF

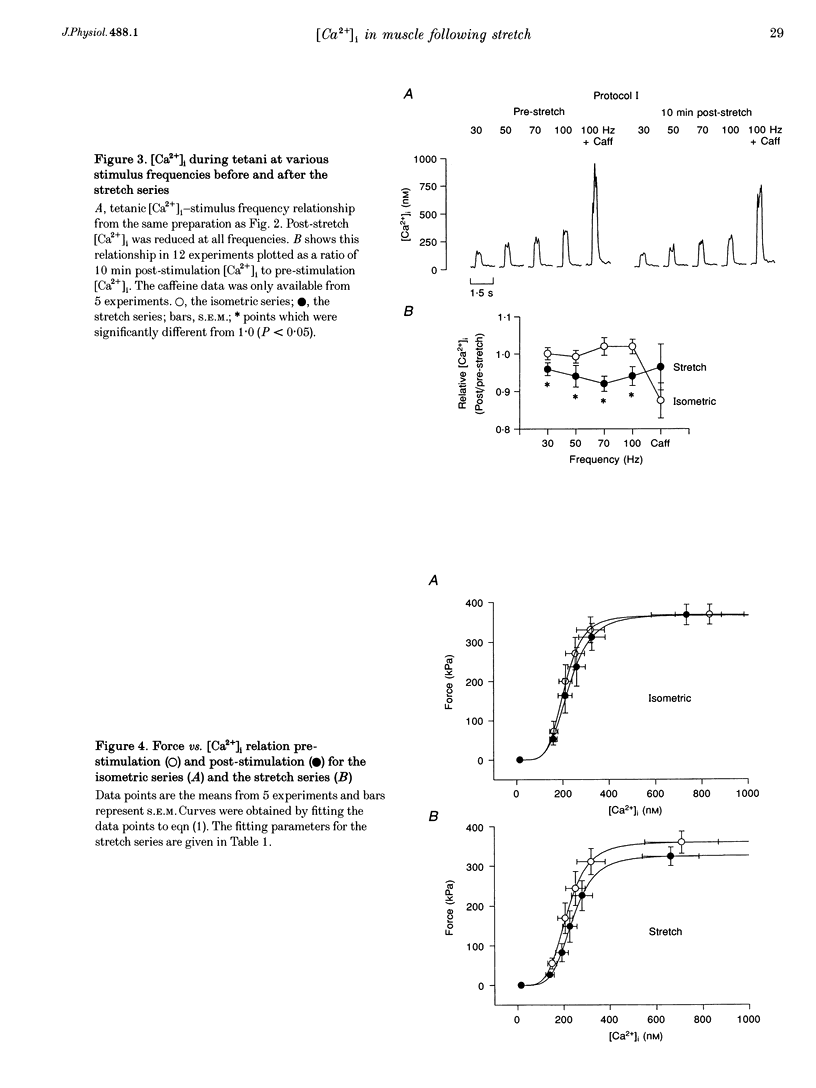



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong R. B., Ogilvie R. W., Schwane J. A. Eccentric exercise-induced injury to rat skeletal muscle. J Appl Physiol Respir Environ Exerc Physiol. 1983 Jan;54(1):80–93. doi: 10.1152/jappl.1983.54.1.80. [DOI] [PubMed] [Google Scholar]
- Armstrong R. B., Warren G. L., Warren J. A. Mechanisms of exercise-induced muscle fibre injury. Sports Med. 1991 Sep;12(3):184–207. doi: 10.2165/00007256-199112030-00004. [DOI] [PubMed] [Google Scholar]
- Balnave C. D., Thompson M. W. Effect of training on eccentric exercise-induced muscle damage. J Appl Physiol (1985) 1993 Oct;75(4):1545–1551. doi: 10.1152/jappl.1993.75.4.1545. [DOI] [PubMed] [Google Scholar]
- Brown L. M., Hill L. Some observations on variations in filament overlap in tetanized muscle fibres and fibres stretched during a tetanus, detected in the electron microscope after rapid fixation. J Muscle Res Cell Motil. 1991 Apr;12(2):171–182. doi: 10.1007/BF01774036. [DOI] [PubMed] [Google Scholar]
- Busch W. A., Stromer M. H., Goll D. E., Suzuki A. Ca 2+ -specific removal of Z lines from rabbit skeletal muscle. J Cell Biol. 1972 Feb;52(2):367–381. doi: 10.1083/jcb.52.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cady E. B., Elshove H., Jones D. A., Moll A. The metabolic causes of slow relaxation in fatigued human skeletal muscle. J Physiol. 1989 Nov;418:327–337. doi: 10.1113/jphysiol.1989.sp017843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies C. T., White M. J. Muscle weakness following eccentric work in man. Pflugers Arch. 1981 Dec;392(2):168–171. doi: 10.1007/BF00581267. [DOI] [PubMed] [Google Scholar]
- Duan C., Delp M. D., Hayes D. A., Delp P. D., Armstrong R. B. Rat skeletal muscle mitochondrial [Ca2+] and injury from downhill walking. J Appl Physiol (1985) 1990 Mar;68(3):1241–1251. doi: 10.1152/jappl.1990.68.3.1241. [DOI] [PubMed] [Google Scholar]
- Duncan C. J. Role of intracellular calcium in promoting muscle damage: a strategy for controlling the dystrophic condition. Experientia. 1978 Dec 15;34(12):1531–1535. doi: 10.1007/BF02034655. [DOI] [PubMed] [Google Scholar]
- Edwards R. H., Hill D. K., Jones D. A., Merton P. A. Fatigue of long duration in human skeletal muscle after exercise. J Physiol. 1977 Nov;272(3):769–778. doi: 10.1113/jphysiol.1977.sp012072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo M. Stretch-induced increase in activation of skinned muscle fibres by calcium. Nat New Biol. 1972 Jun 14;237(76):211–213. doi: 10.1038/newbio237211a0. [DOI] [PubMed] [Google Scholar]
- Faulkner J. A., Jones D. A., Round J. M. Injury to skeletal muscles of mice by forced lengthening during contractions. Q J Exp Physiol. 1989 Sep;74(5):661–670. doi: 10.1113/expphysiol.1989.sp003318. [DOI] [PubMed] [Google Scholar]
- Fridén J., Sjöström M., Ekblom B. A morphological study of delayed muscle soreness. Experientia. 1981 May 15;37(5):506–507. doi: 10.1007/BF01986165. [DOI] [PubMed] [Google Scholar]
- Gilchrist J. S., Wang K. K., Katz S., Belcastro A. N. Calcium-activated neutral protease effects upon skeletal muscle sarcoplasmic reticulum protein structure and calcium release. J Biol Chem. 1992 Oct 15;267(29):20857–20865. [PubMed] [Google Scholar]
- Jones D. A., Newham D. J., Torgan C. Mechanical influences on long-lasting human muscle fatigue and delayed-onset pain. J Physiol. 1989 May;412:415–427. doi: 10.1113/jphysiol.1989.sp017624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M. G., Kovacs L., Simon B. J., Schneider M. F. Decline of myoplasmic Ca2+, recovery of calcium release and sarcoplasmic Ca2+ pump properties in frog skeletal muscle. J Physiol. 1991 Sep;441:639–671. doi: 10.1113/jphysiol.1991.sp018771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lännergren J., Westerblad H. Force decline due to fatigue and intracellular acidification in isolated fibres from mouse skeletal muscle. J Physiol. 1991 Mar;434:307–322. doi: 10.1113/jphysiol.1991.sp018471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCully K. K., Faulkner J. A. Injury to skeletal muscle fibers of mice following lengthening contractions. J Appl Physiol (1985) 1985 Jul;59(1):119–126. doi: 10.1152/jappl.1985.59.1.119. [DOI] [PubMed] [Google Scholar]
- Newham D. J., Jones D. A., Clarkson P. M. Repeated high-force eccentric exercise: effects on muscle pain and damage. J Appl Physiol (1985) 1987 Oct;63(4):1381–1386. doi: 10.1152/jappl.1987.63.4.1381. [DOI] [PubMed] [Google Scholar]
- Newham D. J., McPhail G., Mills K. R., Edwards R. H. Ultrastructural changes after concentric and eccentric contractions of human muscle. J Neurol Sci. 1983 Sep;61(1):109–122. doi: 10.1016/0022-510x(83)90058-8. [DOI] [PubMed] [Google Scholar]
- Stephenson D. G., Williams D. A. Effects of sarcomere length on the force-pCa relation in fast- and slow-twitch skinned muscle fibres from the rat. J Physiol. 1982 Dec;333:637–653. doi: 10.1113/jphysiol.1982.sp014473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren G. L., Lowe D. A., Hayes D. A., Karwoski C. J., Prior B. M., Armstrong R. B. Excitation failure in eccentric contraction-induced injury of mouse soleus muscle. J Physiol. 1993 Aug;468:487–499. doi: 10.1113/jphysiol.1993.sp019783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt I. R., Stephenson D. G. Effects of caffeine on Ca-activated force production in skinned cardiac and skeletal muscle fibres of the rat. Pflugers Arch. 1983 Aug;398(3):210–216. doi: 10.1007/BF00657153. [DOI] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. Changes of myoplasmic calcium concentration during fatigue in single mouse muscle fibers. J Gen Physiol. 1991 Sep;98(3):615–635. doi: 10.1085/jgp.98.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Allen D. G. The influence of intracellular pH on contraction, relaxation and [Ca2+]i in intact single fibres from mouse muscle. J Physiol. 1993 Jul;466:611–628. [PMC free article] [PubMed] [Google Scholar]
- Westerblad H., Duty S., Allen D. G. Intracellular calcium concentration during low-frequency fatigue in isolated single fibers of mouse skeletal muscle. J Appl Physiol (1985) 1993 Jul;75(1):382–388. doi: 10.1152/jappl.1993.75.1.382. [DOI] [PubMed] [Google Scholar]
- Wood S. A., Morgan D. L., Proske U. Effects of repeated eccentric contractions on structure and mechanical properties of toad sartorius muscle. Am J Physiol. 1993 Sep;265(3 Pt 1):C792–C800. doi: 10.1152/ajpcell.1993.265.3.C792. [DOI] [PubMed] [Google Scholar]