Abstract
Objective
Hyperlipidemic patients with end-stage renal disease (ESRD) are at a higher risk of death from cardiovascular diseases, especially from acute myocardial infarction (AMI). Studies on the efficacy of statin therapy (ST) in dialysis patients after percutaneous coronary intervention (PCI) are limited. We examine the mortality associated with ST for these patients.
Methods
From dialysis-dependent hyperlipidemic patients with AMI and receiving PCI in the claims data of National Health Insurance of Taiwan in 2000–2016, we identified a cohort with ST and a cohort without ST matched by propensity score at a 1:1 ratio. Both cohorts were followed up until the end of 2017. All-cause mortalities were examined for both cohorts. Other factors associated with the deaths were also examined.
Results
Among 2642 enrollees in each cohort, over 99 % had hypertension. The all-cause mortality was 23 % lower in the ST cohort than in non-ST cohort (10.8 versus 14.0 per 100 person-years) with an adjusted hazard ratio of 0.77(95 % confidence interval = 0.71–0.84). Patients with comorbidities of diabetes, cerebrovascular accident (CVA) and congestive heart failure (CHF) were also at lower risk. The case-control analysis in the ST cohort showed the estimated risk of death increased with age, and higher for females and patients with peripheral artery disease.
Conclusions
The ST for hyperlipidemic patients with ESRD experiencing AMI undergoing PCI could be benefited with lower all-cause mortality, even for patients with diabetes, hypertension, CVA or CHF.
Keywords: Acute myocardial infarction, All-cause mortality, End-stage renal disease, Gender, Hyperlipidemia, Percutaneous coronary intervention, Statin therapy
Highlights
-
•
We evaluated the efficacy of statin therapy associated with mortality in dialysis patients with AMI receiving PCI.
-
•
The all-cause mortality in dialysis patients with statin therapy decreased for 23 %.
-
•
Females, the elderly and those with peripheral arterial occlusion disease were at increased mortality risk.
-
•
Statin therapy may be considered as part of the treatment regimen in addition to PCI for dialysis patients with AMI.
1. Introduction
Patients with advanced chronic kidney disease (CKD) are at elevated cardiovascular risk as they progress to end-stage renal disease (ESRD) starting dialysis. The mortality from cardiovascular disease (CVD) in these patients was reported to be 10 to 20 times higher than in the general population [1]. Chertow et al. reported that 31–55 % of ESRD patients died within one year after they suffered from an episode of acute myocardial infarction (AMI) [2]. AMI is the most common cause for hospital admission for ESRD patients in Taiwan, and CVD is the most common cause for re-admission within seven days after discharge [3]. For patients suffering from AMI, undergoing revascularization therapy with percutaneous coronary intervention (PCI) and adequate duration of following dual anti-platelet therapy is currently the standard therapy [4,5]. However, for patients suffering from AMI, the proportion of patients receiving the standard therapy was lower in those with ESRD than those without [6].
Statin therapy (ST), an important hyperlipidemia medication for prevention of AMI, has been proved beneficial in reducing CVD events and mortality for general population [7], and for patients with stable coronary disease undergoing PCI [8]. For dialysis patients, previous major randomized controlled trials (RCTs) did not report clinical effectiveness against mortality and therefore ST was not suggested to be initiated [9]. On the contrary, post hoc analyses of the RCTs have shown that the use of statins exerted significant reductions in the risk of cardiac events among hemodialysis patients [10,11]. Besides, recent real-world observational studies reported the treatment effectiveness of ST in chronic dialysis patients with AMI [12,13]. Lin et al. showed that receiving more guidelines-supported medications (statins, antiplatelets, β-blockers and renin–angiotensin–aldosterone system inhibitors) for secondary prevention after AMI was associated with a lower risk of all-cause mortality in hemodialysis patients [14]. However, studies examined the ST effectiveness for dialysis patients who received PCI after AMI episode are limited.
The aim of this study was to assess the association between ST and mortality in hyperlipidemic patients with ESRD who had undergone PCI for AMI during the admission.
2. Materials and methods
2.1. Data source
We conducted this retrospective cohort study using National Health Insurance Research Database (NHIRD) of Taiwan available at the Health and Welfare Data Science Center administered by the Ministry of Health and Welfare (MOHW) of Taiwan. The database contains de-identified claims data of beneficiaries of National Health Insurance, which has covered nearly 99 % of population in Taiwan. The information on demographic status, outpatient and inpatient cares and diagnoses, medications and reimbursements is provided in the database. This study has been reviewed and approved by the Research Ethics Committee at Ditmanson Medical Foundation Chiayi Christian Hospital (CYCH-IRB-2019063), which granted waivers of informed consent from patients because the privacy of insured people had been protected.
2.2. Study population
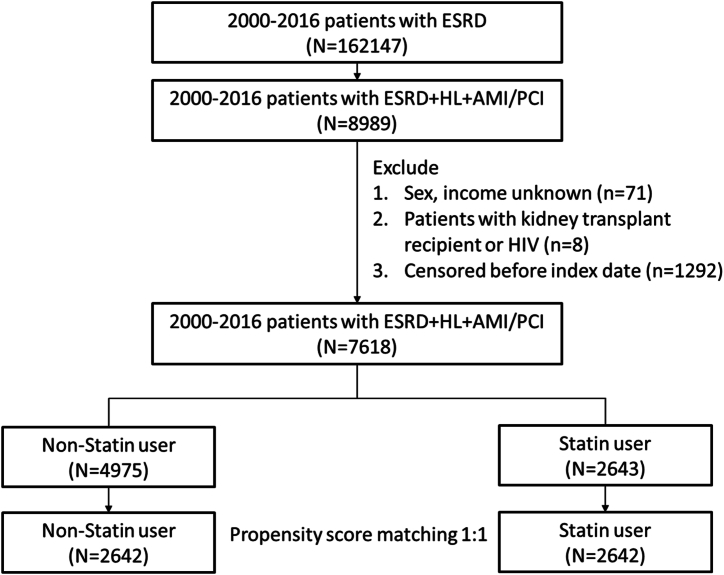
From the claims data of NHIRD in 2000–2016, we identified 162,147 incident ESRD patients aged 20 years and above in catastrophic illness registration cards for ESRD who had received peritoneal dialysis or hemodialysis for 90 days or longer (Fig. 1). Among them, 8989 patients had been diagnosed with hyperlipidemia and AMI, receiving PCI during admission for AMI. The 180th day after the first dialysis date was defined as index date. After excluding patients with unknown sex and income, patients with kidney transplant and patients with human immunodeficiency virus infection, we identified 2643 dialysis patients who had received ST for over 90 days were defined as the statin users. From statin users and non-users, we established a statin users cohort and a non-user cohort with same cohort sizes matched by the propensity score, for which we used logistic regression analysis to estimate the probability of disease status by the baseline age, sex, urbanization, income and comorbidities potentially associated with outcome for each person.
Fig. 1.
Flowchart of study design. Abbreviations: ESRD, end-stage renal disease; HL, hyperlipidemia; AMI, acute myocardial infarction; PCI, percutaneous coronary intervention.
2.3. Definition of outcome and risk factors
This study focused on assessing whether ST was associated with mortality from all causes in the study population compared between statin users and non-users. The covariates that were considered potentially associated with risk estimation included age, sex, urbanization, income, and comorbidities of diabetes mellitus (DM), hypertension, peripheral arterial occlusion disease (PAOD), cerebrovascular accident (CVA), congestive heart failure (CHF), chronic hepatitis B infection (HBV), chronic hepatitis C infection (HCV), and cancers. The comorbidity was defined if it was the primary diagnosis for a hospitalization or it had been diagnosed for at least twice in outpatient cares (See Supplementary Table 1 for diagnosis codes). Both cohorts were followed up until the individuals died, withdrew from the insurance coverage, lost to follow up or the end of 2017. Follow-up person-years for each person and study cohorts, and deceased cases were estimated for estimating the mortality rate.
2.4. Statistical analysis
Distributions of baseline demographic characteristic and comorbidities were compared between cohorts with and without ST. We used t-test to examine means and Chi-square test to examine categorical data. The Kaplan-Meier method was used to estimate and plot the cumulative survival trends for study cohorts and the log-rank test was used to examine the difference. Mortality rates were calculated by covariate and the Cox proportional hazards models were used to calculate crude hazard ratio (cHR) and adjusted hazard ratio (aHR) of all-cause mortality, and related 95 % confidence interval (CI). We calculated aHR after controlling for age, sex, urbanization and incomes in model1 and including all baseline variables in model 2. We further performed a nested case-control analysis for examining factors that might be associated with the deaths in the ST cohort. Logistic regression analysis was used to calculate the odds ratio (OR) of death and 95 % CI. Adjusted ORs were estimated after controlling for the demographic variables in model 1 and after controlling for all baseline variables in model 2. Statistical significance was defined as p < 0.05. All statistical analyses were performed using SAS version 9.4 (SAS-Institute, Cary, NC, USA).
3. Results
The mean age of the statin users and non-users were 66.57 ( ± 10.6) and 66.58 ( ± 10.87), respectively, while the average follow-up periods were 3.60 ( ± 2.42) and 3.71 ( ± 2.79) years. Table 1 shows that the 2642 statin users and 2642 non-users were well matched samples, with near 60 % of men and over 70 % aged 60 years and older. The statin users had higher income than non-users. Both cohorts were prevalent with DM, hypertension and CHF, with a rate of near 90 % or higher.
Table 1.
Baseline characteristics compared between statin users and non-users cohorts.
| Variables | Non-Statin user N = 2642 |
Statin user N = 2642 |
P value |
|---|---|---|---|
| Sex | |||
| Male | 58.1 % | 57.9 % | 0.89 |
| Female | 41.9 % | 40.1 % | |
| Age | |||
| <60 | 27.9 % | 28.2 % | 0.90 |
| 60-79 | 60.4 % | 60.5 % | |
| 80+ | 11.7 % | 11.3 % | |
| Urbanization | |||
| High | 58.5 % | 61.5 % | 0.05 |
| Median | 30.8 % | 29.2 % | |
| Low | 10.7 % | 9.27 % | |
| Income | |||
| <16500 | 27.7 % | 26.3 % | 0.03 |
| 16501-19999 | 42.9 % | 40.9 % | |
| ≥20000 | 29.4 % | 32.8 % | |
| Diabetes | |||
| No | 10.2 % | 10.5 % | 0.72 |
| Yes | 89.8 % | 89.5 % | |
| Hypertension | |||
| No | 0.23 % | 0.34 % | 0.44 |
| Yes | 99.8 % | 99.7 % | |
| PAOD | |||
| No | 74.8 % | 74.4 % | 0.70 |
| Yes | 25.2 % | 25.6 % | |
| HBV | |||
| No | 95.9 % | 96.3 % | 0.43 |
| Yes | 4.09 % | 3.67 % | |
| HCV | |||
| No | 97.0 % | 96.9 | 0.94 |
| Yes | 3.03 % | 3.07 | |
| CVA | |||
| No | 70.5 % | 70.1 % | 0.76 |
| Yes | 29.5 % | 29.9 % | |
| CHF | |||
| No | 5.56 % | 6.09 % | 0.41 |
| Yes | 94.4 % | 93.9 % | |
| Cancer | |||
| No | 87.5 % | 86.9 % | 0.51 |
| Yes | 12.5 % | 13.1 % | |
PAOD, peripheral arterial occlusion disease; HBV, hepatitis B infection; HCV, hepatitis C infection; CVA, cerebrovascular accident; CHF, congestive heart failure.
3.1. All-cause mortality compared between cohorts by covariate
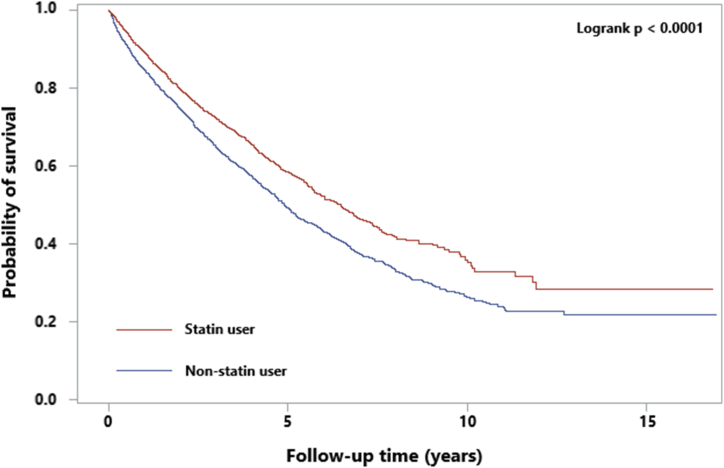
The cumulative survival trends of all causes were significantly higher in the ST cohort than in the non-users (log rank p < 0.0001) (Fig. 2). Table 2 shows that the all-cause mortality rate in the ST cohort was 23 % lower than that in non-users with an aHR of 0.77 (95 % CI = 0.71–0.84) estimated after controlling for all covariates. In the ST cohort, the mortality risk was lower in patients with comorbidity of diabetes, hypertension and congestive heart failure; and for patients without comorbidity of peripheral arterial disease, hepatitis B infection, hepatitis C infection, cancers; and patients with or without CVA. The beneficial outcome seemed greater for patients with DM, hypertension and CHF than patients without the comorbidity.
Fig. 2.
Kaplan–Meier survival curves for all-cause mortality.
Table 2.
All-cause mortalities compared between study cohorts and statin users to non-users hazard ratio by covariates.
| Variables | Non-Statin user N = 2642 |
Statin user N = 2642 |
Crude HR (95 % CI) | Adjusted HR (95 % CI) |
|||||
|---|---|---|---|---|---|---|---|---|---|
| Death No. | person-year | rate | Death No. | person-year | rate | Model 1 | Model 2 | ||
| All | 1371 | 9790 | 14.0 | 1027 | 9507 | 10.8 | 0.77 (0.71–0.83) | 0.77 (0.71–0.84) | 0.77 (0.71–0.84) |
| Sex | |||||||||
| Male | 747 | 5828 | 12.8 | 542 | 5635 | 9.62 | 0.75 (0.67–0.83) | 0.75 (0.67–0.84) | 0.74 (0.67–0.83) |
| Female | 624 | 3962 | 15.7 | 485 | 3872 | 12.5 | 0.79 (0.70–0.89) | 0.80 (0.71–0.91) | 0.80 (0.71–0.91) |
| Age group | |||||||||
| <60 | 327 | 3355 | 9.75 | 241 | 3233 | 7.45 | 0.76 (0.64–0.90) | 0.78 (0.66–0.92) | 0.77 (0.65–0.91) |
| 60-79 | 864 | 5734 | 15.1 | 646 | 5532 | 11.7 | 0.78 (0.70–0.86) | 0.79 (0.71–0.87) | 0.78 (0.71–0.87) |
| 80+ | 180 | 702 | 25.6 | 140 | 742 | 18.9 | 0.74 (0.60–0.93) | 0.74 (0.60–0.93) | 0.74 (0.59–0.92) |
| Urbanization | |||||||||
| Low | 763 | 5811 | 13.1 | 616 | 6026 | 10.2 | 0.77 (0.69–0.86) | 0.77 (0.69–0.85) | 0.77 (0.69–0.85) |
| Median | 453 | 2951 | 15.3 | 312 | 2620 | 11.9 | 0.77 (0.67–0.89) | 0.79 (0.68–0.91) | 0.78 (0.68–0.90) |
| High | 155 | 1029 | 15.1 | 99 | 861 | 11.5 | 0.76 (0.59–0.97) | 0.77 (0.60–1.00) | 0.75 (0.58–0.97) |
| Income | |||||||||
| <16500 | 383 | 2623 | 14.6 | 269 | 2509 | 10.7 | 0.73 (0.63–0.86) | 0.73 (0.62–0.85) | 0.73 (0.63–0.86) |
| 16501-19999 | 604 | 4216 | 14.3 | 451 | 3664 | 12.3 | 0.84 (0.75–0.95) | 0.84 (0.74–0.95) | 0.83 (0.73–0.94) |
| ≥20000 | 384 | 2951 | 13.0 | 307 | 3333 | 9.21 | 0.71 (0.61–0.82) | 0.73 (0.63–0.85) | 0.74 (0.63–0.86) |
| Diabetes | |||||||||
| No | 111 | 1182 | 9.39 | 98 | 1096 | 8.94 | 0.95 (0.72–1.24) | 0.92 (0.70–1.21) | 0.92 (0.70–1.22) |
| Yes | 1260 | 8608 | 14.6 | 929 | 8410 | 11.0 | 0.75 (0.69–0.82) | 0.76 (0.70–0.83) | 0.76 (0.69–0.82) |
| Hypertension | |||||||||
| No | 2 | 25 | 7.93 | 5 | 43 | 11.5 | 1.46 (0.28–7.77) | 4.25 (0.05–353.8) | NA |
| Yes | 1369 | 9765 | 14.0 | 1022 | 9463 | 10.8 | 0.77 (0.71–0.83) | 0.77 (0.71–0.84) | 0.77 (0.71–0.83) |
| PAOD | |||||||||
| No | 1022 | 7585 | 13.5 | 730 | 7342 | 9.94 | 0.73 (0.67–0.80) | 0.74 (0.67–0.81) | 0.74 (0.67–0.81) |
| Yes | 349 | 2205 | 15.8 | 297 | 2165 | 13.7 | 0.87 (0.74–1.01) | 0.88 (0.75–1.02) | 0.87 (0.75–1.02) |
| HBV | |||||||||
| No | 1325 | 9402 | 14.1 | 993 | 9155 | 10.8 | 0.77 (0.70–0.83) | 0.77 (0.71–0.84) | 0.77 (0.71–0.83) |
| Yes | 46 | 388 | 11.89 | 34 | 352 | 9.67 | 0.77 (0.49–1.2) | 0.85 (0.53–1.36) | 0.77 (0.48–1.24) |
| HCV | |||||||||
| No | 1332 | 9531 | 14.0 | 995 | 9245 | 10.8 | 0.77 (0.70–0.83) | 0.77 (0.71–0.84) | 0.77 (0.71–0.84) |
| Yes | 39 | 259 | 15.1 | 32 | 261 | 12.2 | 0.79 (0.5–1.27) | 0.85 (0.51–1.40) | 0.85 (0.51–1.42) |
| Cancer | |||||||||
| No | 1202 | 8733 | 13.8 | 875 | 8389 | 10.4 | 0.75 (0.69–0.82) | 0.76 (0.70–0.83) | 0.76 (0.70–0.83) |
| Yes | 169 | 1057 | 16.0 | 152 | 1118 | 13.6 | 0.85 (0.69–1.06) | 0.83 (0.67–1.04) | 0.84 (0.67–1.05) |
| CVA | |||||||||
| No | 928 | 7219 | 12.8 | 701 | 6910 | 10.1 | 0.78 (0.71–0.87) | 0.79 (0.72–0.87) | 0.79 (0.71–0.87) |
| Yes | 443 | 2570 | 17.2 | 326 | 2596 | 12.6 | 0.73 (0.63–0.84) | 0.73 (0.64–0.85) | 0.74 (0.64–0.85) |
| CHF | |||||||||
| No | 58 | 631 | 9.19 | 52 | 678 | 7.67 | 0.85 (0.58–1.23) | 0.95 (0.65–1.41) | 0.99 (0.66–1.47) |
| Yes | 1313 | 9159 | 14.3 | 975 | 8828 | 11.0 | 0.76 (0.70–0.83) | 0.77 (0.71–0.83) | 0.76 (0.70–0.83) |
PAOD, peripheral arterial occlusion disease; HBV, hepatitis B infection; HCV, hepatitis C infection; CVA, cerebrovascular accident; CHF, congestive heart failure; HR, hazard ratio; CI, confidence interval.
Adjusted model 1: multivariable analysis including age, sex, urbanization, incomes.
Adjusted model 2: multivariable analysis including age, sex, urbanization, incomes and all comorbidities.
3.2. Nested case-control analysis in the statin cohort
In the nested case-control analysis model as in Table 3, we found an increased risk of all-cause mortality in female patients (aOR = 1.30, 95 % CI = 1.11–1.54), the elderly patients (with aORs of 1.32 (95 % CI = 1.09–1.59) for aged 60–79 years old and of 1.66 (95 % CI = 1.25–2.20) for ≥80 years old, compared to patients aged <60 years old), and in patients with PAOD (aOR = 1.26, 95 % CI = 1.05–1.51).
Table 3.
Nested case-control analysis for all-cause mortality from chronic dialysis patients with PCI in the statin cohort.
| Death |
Crude |
Adjusted OR (95 % CI) |
||||
|---|---|---|---|---|---|---|
| No (N = 1615) | Yes (N = 1027) | All (N = 2642) | OR (95 % CI) | Model 1 | Model 2 | |
| Sex | ||||||
| Male | 988 | 542 | 1530 | Ref. | Ref. | Ref. |
| Female | 627 | 485 | 1112 | 1.41 (1.20–1.65) | 1.32 (1.13–1.56) | 1.30 (1.11–1.54) |
| Age group | ||||||
| <60 | 504 | 241 | 745 | Ref. | Ref. | Ref. |
| 60-79 | 952 | 646 | 1598 | 1.42 (1.18–1.70) | 1.36 (1.13–1.64) | 1.32 (1.09–1.59) |
| 80+ | 159 | 140 | 299 | 1.84 (1.40–2.42) | 1.73 (1.31–2.28) | 1.66 (1.25–2.20) |
| Urbanization | ||||||
| Low | 1009 | 616 | 1625 | Ref. | Ref. | Ref. |
| Median | 460 | 312 | 772 | 1.11 (0.93–1.32) | 1.10 (0.92–1.32) | 1.09 (0.91–1.30) |
| High | 146 | 99 | 245 | 1.11 (0.84–1.46) | 1.04 (0.78–1.38) | 1.04 (0.78–1.38) |
| Income | ||||||
| <16500 | 426 | 269 | 695 | Ref. | Ref. | Ref. |
| 16501-19999 | 630 | 451 | 1081 | 1.13 (0.93–1.38) | 1.09 (0.90–1.33) | 1.09 (0.91–1.30) |
| ≥20000 | 559 | 307 | 866 | 0.87 (0.71–1.07) | 0.91 (0.74–1.12) | 1.04 (0.78–1.38) |
| DM | ||||||
| No | 179 | 98 | 277 | Ref. | Ref. | |
| Yes | 1436 | 929 | 2365 | 1.18 (0.91–1.53) | 1.14 (0.87–1.49) | |
| HTN | ||||||
| No | 4 | 5 | 9 | Ref. | Ref. | |
| Yes | 1611 | 1022 | 2633 | 0.51 (0.14–1.89) | 0.41 (0.11–1.57) | |
| PAOD | 0 (0–0) | |||||
| No | 1235 | 730 | 1965 | Ref. | Ref. | |
| Yes | 380 | 297 | 677 | 1.32 (1.11–1.58) | 1.26 (1.05–1.51) | |
| HBV | ||||||
| No | 1552 | 993 | 2545 | Ref. | Ref. | |
| Yes | 63 | 34 | 97 | 0.84 (0.55–1.29) | 0.93 (0.60–1.43) | |
| HCV | ||||||
| No | 1566 | 995 | 2561 | Ref. | Ref. | |
| Yes | 49 | 32 | 81 | 1.03 (0.65–1.62) | 1.01 (0.64–1.60) | |
| Cancer | ||||||
| No | 1420 | 875 | 2295 | Ref. | Ref. | |
| Yes | 195 | 152 | 347 | 1.27 (1.01–1.59) | 1.21 (0.96–1.53) | |
| CVA | ||||||
| No | 1152 | 701 | 1853 | Ref. | Ref. | |
| Yes | 463 | 326 | 789 | 1.16 (0.98–1.37) | 1.08 (0.91–1.29) | |
| CHF | ||||||
| No | 109 | 52 | 161 | Ref. | Ref. | |
| Yes | 1506 | 975 | 2481 | 1.36 (0.97–1.91) | 1.20 (0.85–1.70) | |
PAOD, peripheral arterial occlusion disease; HBV, hepatitis B infection; HCV, hepatitis C infection; CVA, cerebrovascular accident; CHF, congestive heart failure; OR, odds ratio; CI, confidence interval.
Adjusted model 1: multivariable analysis including age, sex, urbanization, incomes.
Adjusted model 2: multivariable analysis including age, sex, urbanization, incomes and all comorbidities.
4. Discussion
With propencity score matching, we established well matched cohorts with and without ST to examine the all-cause mortality in hyperlipidemic dialysis-dependent patients who had experienced AMI and revascularization with PCI. The ST is truly associated with 23 % decreased mortality. The ST effectiveness seems to increase with age. In the ST cohort, females, the elderly and patients with PAOD were at higher risk of mortality.
The benefit outcome from ST in our study is consistent with several previous studies which also reported that ST was associated with reduced mortality risk in dialysis patients who experienced AMI or other critical conditions. We recently reported that the 3624 ESRD patients with ST had an aHR of 0.68compared with patients without ST [15]. Chung et al. reported that the all-cause mortality was reduced for 30 % and 24 % in 1-year and 4-year of follow-up in 1580 dialysis patients who had received moderate or high intensity ST within 30 days after the AMI episodes [12]. In a Korean population-based study, Jung et al. reported that hemodialysis patients with continued ST had the hazards of all-cause mortality 52 % lower than non-users had [16]. A case-control study using Taiwan's insurance claims data also confirmed the ST is associated with 11 % reduced risk of all-cause mortality in 6354 ESRD patients with AMI, though higher PCI prevalence was noted in the ST group (61.24 % in statin users and 38.39 % in non-users) [13].
While PCI offers substantial clinical benefits for patients with AMI, dialysis patients face markedly elevated risks of in-hospital death and one-year mortality post-PCI, 7 to 10 times higher than those with CKD stage 1 [17]. Notably, individuals with ESRD are often excluded from randomized trials assessing revascularization outcomes [18]. Studies have suggested that PCI for AMI in dialysis patients is linked to lower mortality risks than patients receiving medical management [2,19]. However, the therapeutic effect of ST at PCI for patients with renal impairment remained controversial. In a study of 548 patients with advanced renal dysfunction (eGFR <30 mL/min/1.73 m2) undergoing PCI after AMI, no significant benefits in all-cause mortality for those with ST were observed [20]. In a research cohort of 2306 CKD patients (eGFR <60 mL/min/1.73 m2) undergoing PCI, Dasari et al. found that statins prescribed at discharge significantly improved one-year mortality (adjusted HR: 0.55) and the composite endpoint of death, MI, and repeated revascularization [21]. Nevertheless, this association was not significant in the subgroup of 165 dialysis patients, possibly due to the small sample size [21].
Conversely, studies from East Asia on patients with ESRD under dialysis post-PCI indicated that statins might have a prognostic benefit in reducing the risk of all-cause mortality, aligning with our research. Kim et al. reported a 46 % risk reduction in a composite of MI, CVA, and all-cause mortality in 150 chronic hemodialysis patients receiving ST after PCI in Korea [22]. A study by Funamizu et al. in Japan involving 201 hemodialysis patients demonstrated that statin use at PCI was significantly associated with reduced cardiovascular events (HR: 0.43) and all-cause mortality (HR: 0.50), suggesting that the benefit might not solely be attributed to its LDL cholesterol lowering property [23].
DM, hypertension and CHF were highly prevalent comobidities in our study population. Our stratified analyses revealed that individuals with these comorbidities exhibited notable connections with decreased all-cause mortality. The consistent beneficial associations with ST for patients with these comorbidities are likely due to the large size and multicollinearity with hyperlipidemia. In a prospective study spanning three years, Horikoshi et al. similarly indicated the efficacy of statins in averting major adverse cardiac events (adjusted HR: 0.30) among hemodialysis patients who underwent PCI. Furthermore, the weighted stratification analyses uncovered a significant and advantageous association with clinical factors including DM and hypertension [24].
The potential mechanism for the reduced all-cause mortality associated with ST in dialysis patients with AMI who underwent PCI can be attributed to several factors. Systemic inflammation and endothelial dysfunction are commonly observed in patients with AMI and those undergoing dialysis. Statins have anti-inflammatory and anti-oxidation properties, and can improve endothelial function. Statins help stabilizing atherosclerotic plaques and refer to the ability of blood vessels to dilate and maintain adequate blood flow [25,26]. Besides, statin may improve renal anemia in ESRD patients receiving hemodialysis and regular erythropoietin-stimulating agents by reducing inflammation [27]. Therefore, ST could be considered to provide additional benefits in managing cardiovascular disease in this population.
However, several other mechanisms have been proposed to explain the clinically inconsistency associated statin use for dialysis patients with AMI. Traditional risk factors alone cannot fully account for these outcomes. Studies have identified contributing factors such as anemia [28], imbalances in calcium and phosphorus levels [29], and parathyroid hormone abnormalities, which may have a greater impact on the incidence of CVD in patients with CKD. Additionally, increased levels of oxidative stress, proinflammatory cytokines, and uremic toxins such as p-cresol and indoxyl sulfate [30] have been described as endotheliotoxins that contribute to CVD pathogenesis or are associated with vascular calcification [31]. Also, Di-(2-ethylhexyl) phthalate (DEHP), the plasticizer extensively used in medical tubes and blood storage bags, may limit the pleiotropic effects of statins in patients undergoing dialysis [32].
This study has the advantages of using the nationally representative data with a relatively large sample size. To reduce selection bias, study cohorts were established and analyzed after propensity score matching. The present study demonstrated a convincing benefit. In addition to the present study, a recent study also reported that patients with dialysis are benefited from ST [15]. With the positive findings and potential implications, our findings may encourage clinicians to prescribe ST for dialysis patients with hyperlipidemia.
There were some limitations in this study. First, the information on life styles and the underlying cause of ESRD was unavailable in the database. In addition, data of the severity of AMI lesions (such as numbers of diseased vessels), blood supply recovery after PCI, serum cholesterol levels, and cardiac/coronary images (such as left ventricular ejection fraction) were also not available in a medical claims database. We were unable to adjust for all these factors. Furthermore, the present study was based on a homogeneous Asian population, and the generalizability of findings to other ethnic groups may be limited. Given the limitations inherent to the retrospective observational design and the complex condition of the disorders, it is not possible to conclude with certainty that ST causes better outcomes. Therefore, more studies including randomized controlled trials are necessary to confirm the findings.
5. Conclusion
ST prescribed for hyperlipidemia patients with dialysis seemed to have the benefit of lower all-cause mortality in those developed AMI undergoing PCI than those not prescribed statin. According to our results, we may consider statin therapy for hyperlipidemia patients with dialysis patients, particularly for those with comorbidity of DM, hypertension.
CRediT authorship contribution statement
Yi-Ting Yeh: Writing – review & editing, Writing – original draft, Methodology, Conceptualization. Fung-Chang Sung: Writing – review & editing, Writing – original draft, Resources, Methodology. Ching-Fang Tsai: Writing – review & editing, Resources, Methodology, Conceptualization. Chih-Cheng Hsu: Writing – review & editing, Supervision, Resources. Wen-Chen Tsai: Writing – review & editing, Supervision, Resources. Yueh-Han Hsu: Writing – review & editing, Writing – original draft, Supervision, Methodology, Conceptualization.
Ethics statement
This study was approved by the Research Ethics Committee at Ditmanson Medical Foundation Chiayi Christian Hospital (CYCH-IRB-2019063). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Data availability statement
The original database was available at the Health and Welfare Data Science Center administered by the Ministry of Health and Welfare of Taiwan. Researcher has to apply for permit to use the NHIRD after the IRB approval. The approved proposal may have an approximately 2-year quota to make appointments for conducting data analysis at the center.
Fundings
This study is supported in part by Taiwan Ministry of Health and Welfare Clinical Trial Center (MOHW110-TDU-B-212-124004), Ministry of Science and Technology (MOST110-2321-B-039-003), and China Medical University Hospital (DMR-111-228), and Ditmanson Medical Foundation Chiayi Christian Hospital (R-109-17). The funders have no role in the study design, data collection and analysis, the decision to publish, or preparation of the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We are grateful to Health Data Science Center, China Medical University Hospital, and Ditmanson Medical Foundation Chiayi Christian Hospital for providing administrative, technical and funding support for using the NHIRD.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.heliyon.2024.e39906.
Appendix A. Supplementary data
The following is the supplementary data to this article:
References
- 1.Foley R.N., Parfrey P.S., Sarnak M.J. Clinical epidemiology of cardiovascular disease in chronic renal disease. Am. J. Kidney Dis. 1998;32:S112–S119. doi: 10.1053/ajkd.1998.v32.pm9820470. [DOI] [PubMed] [Google Scholar]
- 2.Chertow G.M., Normand S.-L.T., Silva L.R., McNeil B.J. Survival after acute myocardial infarction in patients with end-stage renal disease: results from the cooperative cardiovascular project. Am. J. Kidney Dis. 2000;35:1044–1051. doi: 10.1016/s0272-6386(00)70038-2. [DOI] [PubMed] [Google Scholar]
- 3.Taiwan Society of Nephrology . In: 2021 Kidney Disease in Taiwan, Annual Report. Hwang S.J., editor. Taipei; Taiwan: 2021. National Health research institute; pp. 115–135. (Taiwan Society of Nephrology). (In Chinese) [Google Scholar]
- 4.Neumann F.-J., Sousa-Uva M., Ahlsson A., Alfonso F., Banning A.P., Benedetto U., et al. ESC/EACTS Guidelines on myocardial revascularization. Eur. Heart J. 2018;40:87–165. 2019. [Google Scholar]
- 5.Lawton J.S., Tamis-Holland J.E., Bangalore S., Bates E.R., Beckie T.M., Bischoff J.M., et al. ACC/AHA/SCAI guideline for coronary artery revascularization: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;145:e4–e17. doi: 10.1161/CIR.0000000000001039. 2022. [DOI] [PubMed] [Google Scholar]
- 6.Saad M., Karam B., Faddoul G., Douaihy Y.E., Yacoub H., Baydoun H., et al. Is kidney function affecting the management of myocardial infarction? A retrospective cohort study in patients with normal kidney function, chronic kidney disease stage III-V, and ESRD. Int. J. Nephrol. Renovascular Dis. 2016;9:5–10. doi: 10.2147/IJNRD.S91567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Visseren F.L.J., Mach F., Smulders Y.M., Carballo D., Koskinas K.C., Bäck M., et al. ESC Guidelines on cardiovascular disease prevention in clinical practice. Eur. Heart J. 2021;42:3227–3337. doi: 10.1093/eurheartj/ehab484. 2021. [DOI] [PubMed] [Google Scholar]
- 8.Wu H.P., Yang F.C., Lin H.D., Cai C.-Z., Chuang M.-J., Chiang K.F., et al. Association between statin therapy and long-term clinical outcomes in patients with stable coronary disease undergoing percutaneous coronary intervention. Sci. Rep. 2024;14 doi: 10.1038/s41598-024-63598-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Palmer S.C., Navaneethan S.D., Craig J.C., Johnson D.W., Perkovic V., Nigwekar S.U., et al. HMG CoA reductase inhibitors (statins) for dialysis patients. Cochrane Database Syst. Rev. 2013;9 doi: 10.1002/14651858.CD004289.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holdaas H., Holme I., Schmieder R.E., Jardine A.G., Zannad F., Norby G.E., et al. Rosuvastatin in diabetic hemodialysis patients. J. Am. Soc. Nephrol. 2011;22:1335–1341. doi: 10.1681/ASN.2010090987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marz W., Genser B., Drechsler C., Krane V., Grammer T.B., Ritz E., et al. Atorvastatin and low-density lipoprotein cholesterol in type 2 diabetes mellitus patients on hemodialysis. Clin. J. Am. Soc. Nephrol. 2011;6:1316–1325. doi: 10.2215/CJN.09121010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chung C.M., Lin M.S., Chang C.H., Cheng H.-W., Chang S.-T., Wang P.-C., et al. Moderate to high intensity statin in dialysis patients after acute myocardial infarction: a national cohort study in Asia. Atherosclerosis. 2017;267:158–166. doi: 10.1016/j.atherosclerosis.2017.09.018. [DOI] [PubMed] [Google Scholar]
- 13.Kuo F.Y., Huang W.C., Tang P.L., Cheng C.-C., Chiang C.-H., Lin H.-C., et al. Impact of statin on long-term outcome among patients with end-stage renal disease with acute myocardial infarction (AMI): a nationwide case-control study. Postgrad Med J. 2021;97:299–305. doi: 10.1136/postgradmedj-2019-137292. [DOI] [PubMed] [Google Scholar]
- 14.Lin T.Y., Hsieh T.H., Hung S.C. Association of secondary prevention medication use after myocardial infarction with mortality in hemodialysis patients. Clin Kidney J. 2022;15:2135–2143. doi: 10.1093/ckj/sfac170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sung F.C., Jong Y.C., Muo C.H., Hsu C.-C., Tsai W.-C., Hsu Y.-H. Statin therapy for hyperlipidemic patients with chronic kidney disease and end-stage renal disease: a retrospective cohort study based on 925,418 adults in taiwan. Front. Pharmacol. 2022;3 doi: 10.3389/fphar.2022.815882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jung J., Bae G.H., Kang M., Kim S.W., Lee D.H. Statins and all-cause mortality in patients undergoing hemodialysis. J. Am. Heart Assoc. 2020;9 doi: 10.1161/JAHA.119.014840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Limpijankit T., Chandavimol M., Srimahachota S., et al. Dose-dependent effect of impaired renal function on all-cause mortality in patients following percutaneous coronary intervention. Clin. Cardiol. 2022;45:882–891. doi: 10.1002/clc.23877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Coca S.G., Krumholz H.M., Garg A.X., Parikh C.R. Underrepresentation of renal disease in randomized controlled trials of cardiovascular disease. JAMA. 2006;296:1377–1384. doi: 10.1001/jama.296.11.1377. [DOI] [PubMed] [Google Scholar]
- 19.Kawsara A., Sulaiman S., Mohamed M., Paul T.K., Kashani K.B., Boobes K., et al. Treatment effect of percutaneous coronary intervention in dialysis patients with ST-elevation myocardial infarction. Am. J. Kidney Dis. 2022;79:832–840. doi: 10.1053/j.ajkd.2021.08.023. [DOI] [PubMed] [Google Scholar]
- 20.Kim J.S., Kim W., Park J.Y., Woo J.S., Lee T.W., Ihm C.G., et al. Effects of statin therapy on clinical outcomes after acute myocardial infarction in patients with advanced renal dysfunction: a propensity score-matched analysis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0183059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dasari T.W., Cohen D.J., Kleiman N.S., Keyes M.J., Yen C.-H., Hanna E.B., et al. Statin therapy in patients with chronic kidney disease undergoing percutaneous coronary intervention (from the Evaluation of Drug Eluting Stents and Ischemic Events Registry) Am. J. Cardiol. 2014;113:621–625. doi: 10.1016/j.amjcard.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 22.Kim S.H., Jeong H.Y., Yang D.H., Kim J., Lee S.-Y. Beneficial effect of statins in patients receiving chronic hemodialysis following percutaneous coronary intervention: a nationwide retrospective cohort study. Sci. Rep. 2018;8:9692. doi: 10.1038/s41598-018-27941-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Funamizu T., Iwata H., Chikata Y., Doi S., Endo H., Wada H., et al. A prognostic merit of statins in patients with chronic hemodialysis after percutaneous coronary intervention-A 10-year follow-up study. J. Clin. Med. 2022;11:390. doi: 10.3390/jcm11020390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horikoshi T., Nakamura T., Yoshizaki T., Nakamura J., Makino A., Saito Y., et al. Stratification analysis of statin effect on major adverse cardiac events after percutaneous coronary intervention in patients on hemodialysis. J. Cardiovasc. Pharmacol. 2022;79:168–176. doi: 10.1097/FJC.0000000000001152. [DOI] [PubMed] [Google Scholar]
- 25.Laufs U., Fata V.L., Plutzky J., Liao J.K. Upregulation of endothelial nitric oxide synthase by HMG CoA reductase inhibitors. Circulation. 1998;97:1129–1135. doi: 10.1161/01.cir.97.12.1129. [DOI] [PubMed] [Google Scholar]
- 26.Geng J., Xu H., Yu X., Xu G., Cao H., Lin G., et al. Rosuvastatin protects against oxidized low-density lipoprotein-induced endothelial cell injury of atherosclerosis in vitro. Mol. Med. Rep. 2019;19:432–440. doi: 10.3892/mmr.2018.9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tsai M.H., Su F.Y., Chang H.Y., Su P.-C., Chiu L.-Y., Nowicki M., et al. The effect of statin on anemia in patients with chronic kidney disease and end-stage kidney disease: a systematic review and meta-analysis. J Pers Med. 2022;12:1175. doi: 10.3390/jpm12071175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Francisco A.L., Stenvinkel P., Vaulont S. Inflammation and its impact on anaemia in chronic kidney disease: from haemoglobin variability to hyporesponsiveness. NDT Plus. 2009;2:i18–i26. doi: 10.1093/ndtplus/sfn176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laucyte-Cibulskiene A., Petraviciute M., Gudynaite M., Gumbys L., Valanciene D., Galiauskiene K., et al. Mismatch between stiffness in elastic and muscular arteries as a predictor of vascular calcification in dialysis patients. Aging Clin. Exp. Res. 2018;30:375–382. doi: 10.1007/s40520-017-0787-7. [DOI] [PubMed] [Google Scholar]
- 30.Tsai M.H., Chang C.H., Liou H.H., Fang Y.-W. Inverted U-curve association between serum indoxyl sulfate levels and cardiovascular events in patients on chronic hemodialysis. J. Clin. Med. 2021;10:744. doi: 10.3390/jcm10040744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Opdebeeck B., D'Haese P.C., Verhulst A. Molecular and cellular mechanisms that induce arterial calcification by indoxyl sulfate and P-cresyl sulfate. Toxins. 2020;12:58. doi: 10.3390/toxins12010058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Guo B.C., Kuo K.L., Chen C.H., Chen S.-L., Tsou T.C., Lee T.-S. Di-(2-ethylhexyl) phthalate limits the pleiotropic effects of statins in chronic kidney disease patients undergoing dialysis and endothelial cells. Environ Pollut. 2020;267 doi: 10.1016/j.envpol.2020.115548. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The original database was available at the Health and Welfare Data Science Center administered by the Ministry of Health and Welfare of Taiwan. Researcher has to apply for permit to use the NHIRD after the IRB approval. The approved proposal may have an approximately 2-year quota to make appointments for conducting data analysis at the center.