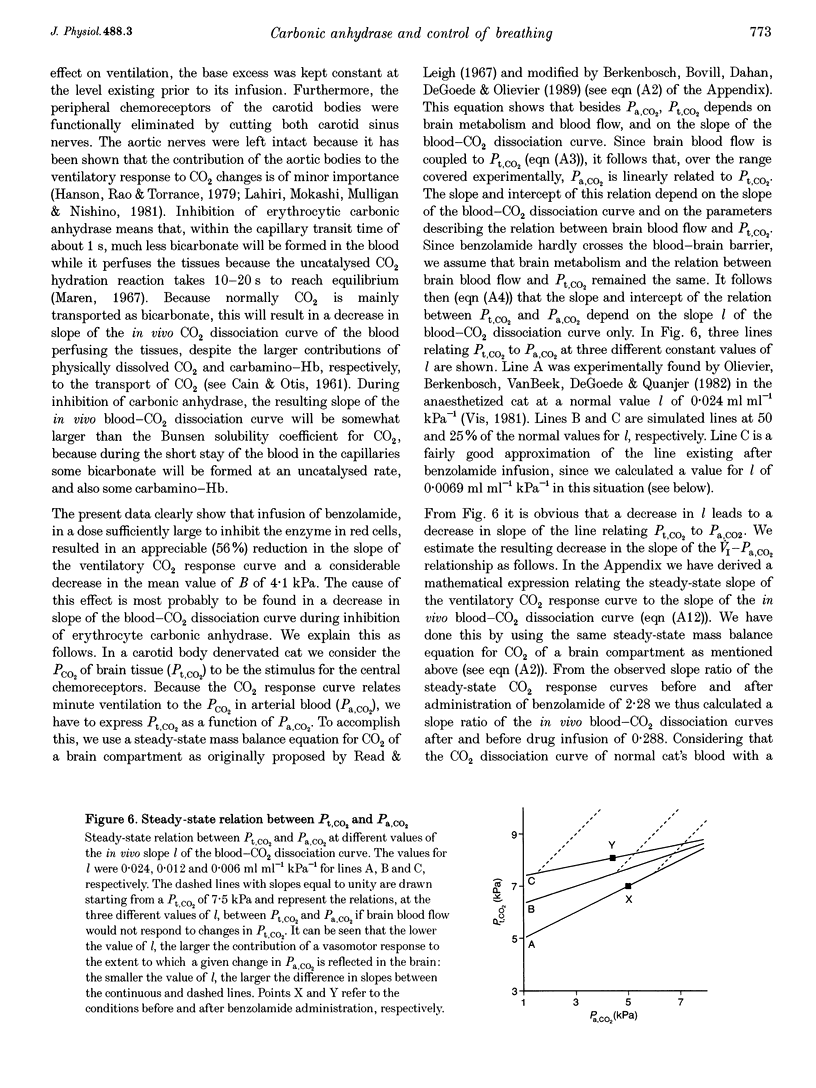
Abstract
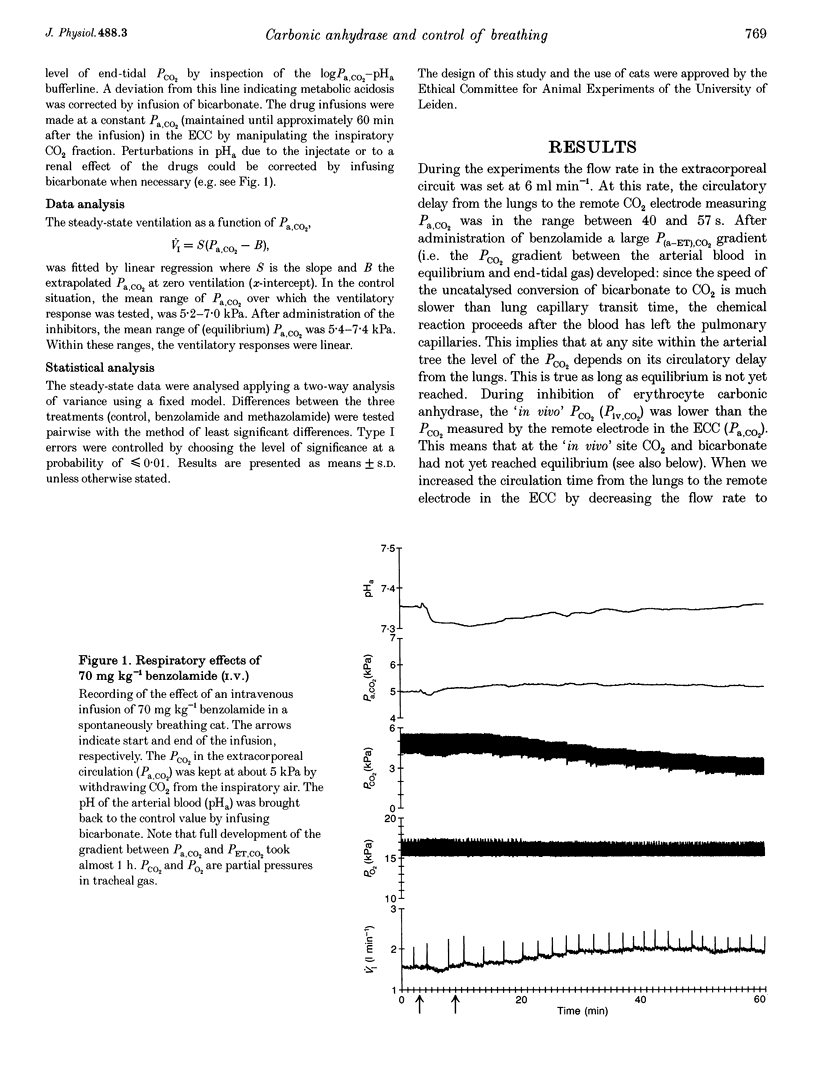
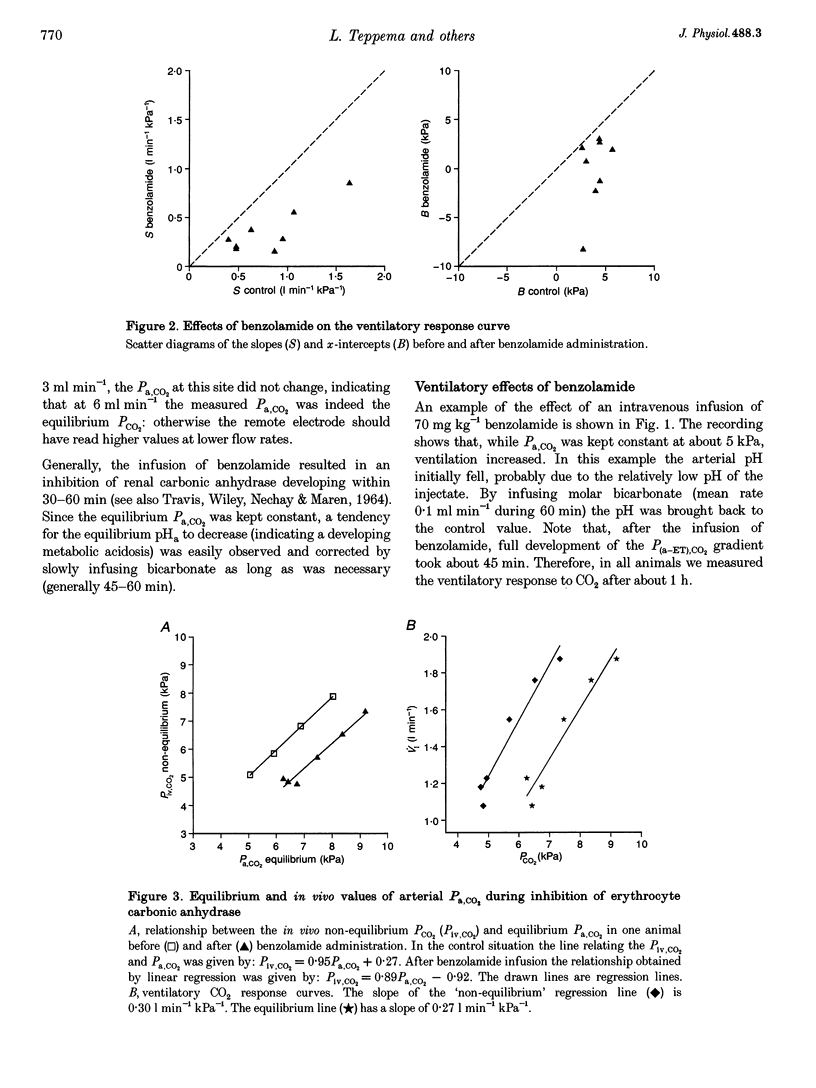
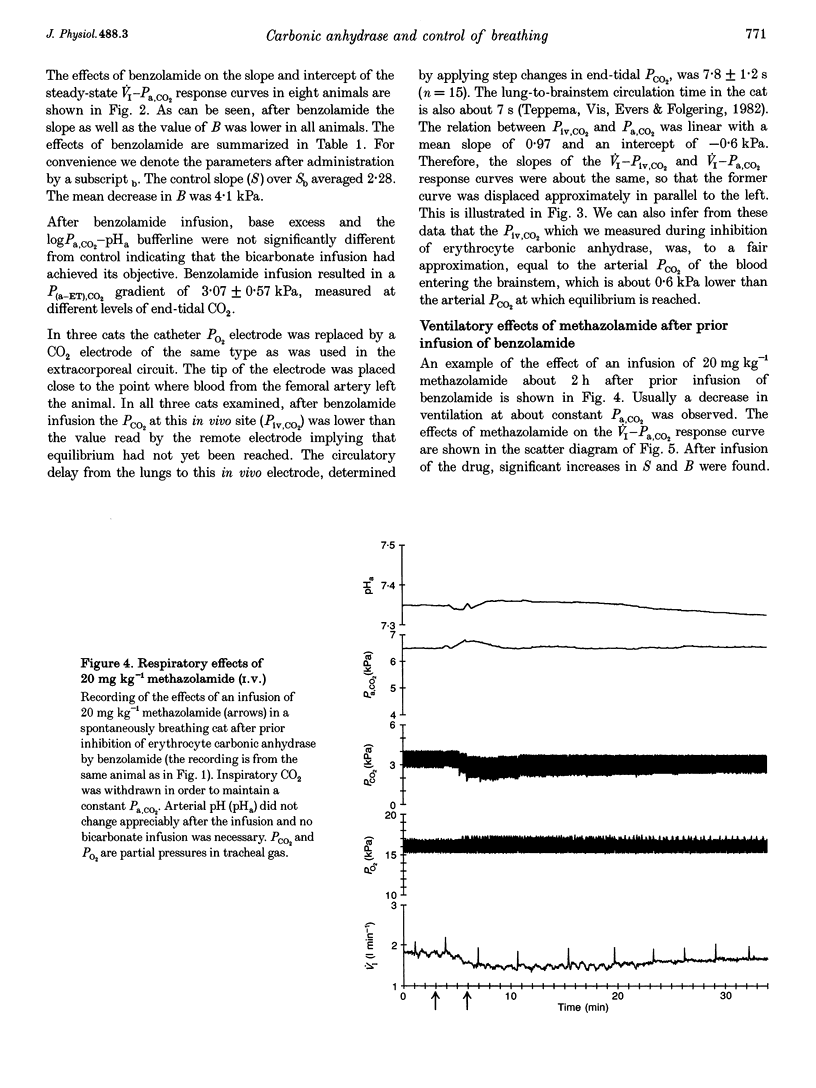
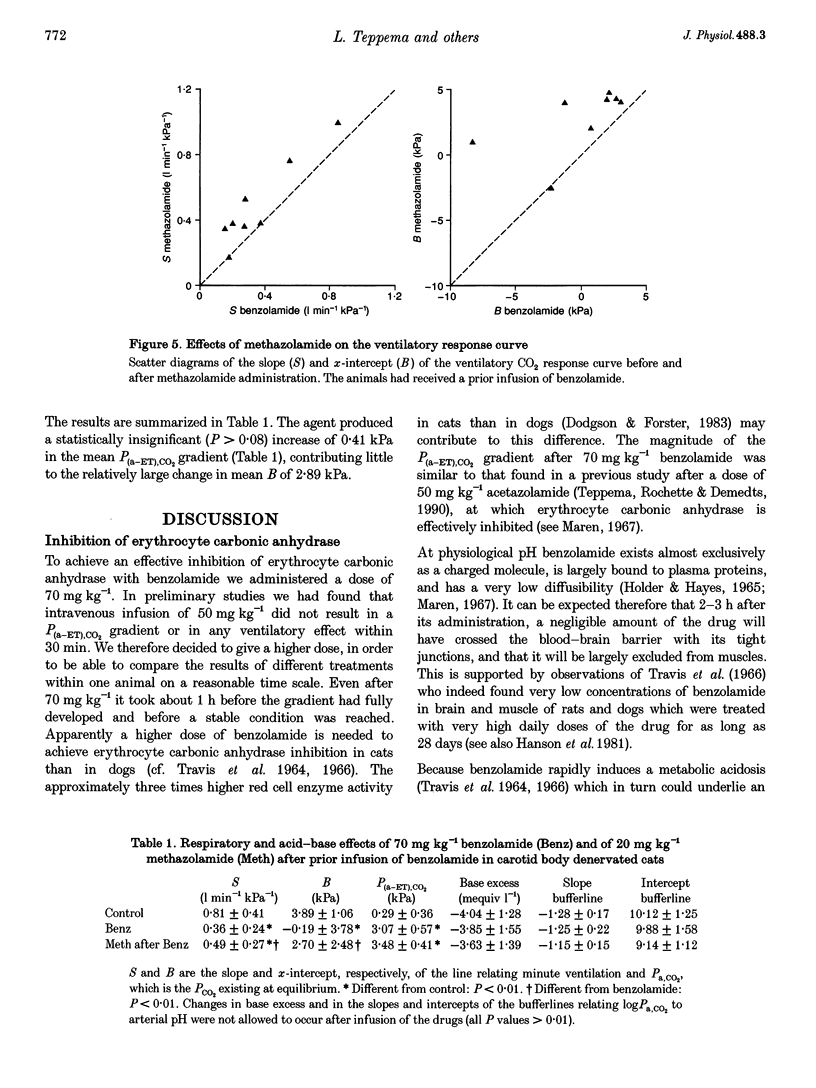
1. The effect of inhibition of erythrocyte carbonic anhydrase on the ventilatory response to CO2 was studied by administering benzolamide (70 mg kg-1, i.v.), an inhibitor which does not cross the blood-brain barrier, to carotid body denervated cats which were anaesthetized with chloralose-urethane. 2. In the same animals the effect on the ventilatory response to CO2 of subsequent inhibition of central nervous system (CNS) carbonic anhydrase was studied by infusing methazolamide (20 mg kg-1), an inhibitor which rapidly penetrates into brain tissue. 3. The results show that inhibition of erythrocyte carbonic anhydrase by benzolamide leads to a decrease in the slope of the normoxic CO2 response curve, and a decrease of the extrapolated arterial PCO2 at zero ventilation. 4. Inhibition of CNS carbonic anhydrase by methazolamide results in an increase in slope and alpha-intercept of the ventilatory CO2 response curve. 5. Using a mass balance equation for CO2 of a brain compartment, it is argued that inhibition of erythrocyte carbonic anhydrase results in a decrease in slope of the in vivo CO2 dissociation curve, which can explain the effects of benzolamide. 6. The changes in slope and intercept induced by methazolamide are discussed in relation to effects on neurones containing carbonic anhydrase, which may include central chemoreceptors.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. M., Johnson N. L. Inhibiting carbonic anhydrase in brain tissue increases the respiratory response to rebreathing CO2. Brain Res. 1990 Jun 11;519(1-2):23–28. doi: 10.1016/0006-8993(90)90056-h. [DOI] [PubMed] [Google Scholar]
- Andreatta-van Leyen S., Averill D. B., Guertzenstein P. G. Cardiorespiratory effects induced by acetazolamide on the ventromedullary surface of the cat. J Physiol. 1990 Feb;421:171–184. doi: 10.1113/jphysiol.1990.sp017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkenbosch A., Bovill J. G., Dahan A., DeGoede J., Olievier I. C. The ventilatory CO2 sensitivities from Read's rebreathing method and the steady-state method are not equal in man. J Physiol. 1989 Apr;411:367–377. doi: 10.1113/jphysiol.1989.sp017578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CAIN S. M., OTIS A. B. Carbon dioxide transport in anesthetized dogs during inhibition of carbonic anhydrase. J Appl Physiol. 1961 Nov;16:1023–1028. doi: 10.1152/jappl.1961.16.6.1023. [DOI] [PubMed] [Google Scholar]
- Coates E. L., Li A. H., Nattie E. E. Acetazolamide on the ventral medulla of the cat increases phrenic output and delays the ventilatory response to CO2. J Physiol. 1991 Sep;441:433–451. doi: 10.1113/jphysiol.1991.sp018760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coates E. L., Li A., Nattie E. E. Widespread sites of brain stem ventilatory chemoreceptors. J Appl Physiol (1985) 1993 Jul;75(1):5–14. doi: 10.1152/jappl.1993.75.1.5. [DOI] [PubMed] [Google Scholar]
- Dahan A., Berkenbosch A., DeGoede J., Olievier I. C., Bovill J. G. On a pseudo-rebreathing technique to assess the ventilatory sensitivity to carbon dioxide in man. J Physiol. 1990 Apr;423:615–629. doi: 10.1113/jphysiol.1990.sp018043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J. B., Lawing W. L., Millhorn D. E. CO2 decreases membrane conductance and depolarizes neurons in the nucleus tractus solitarii. Exp Brain Res. 1989;76(3):656–661. doi: 10.1007/BF00248922. [DOI] [PubMed] [Google Scholar]
- Dodgson S. J., Forster R. E., 2nd Carbonic anhydrase activity of intact erythrocytes from seven mammals. J Appl Physiol Respir Environ Exerc Physiol. 1983 Oct;55(4):1292–1298. doi: 10.1152/jappl.1983.55.4.1292. [DOI] [PubMed] [Google Scholar]
- Effros R. M., Chang R. S., Silverman P. Acceleration of plasma bicarbonate conversion to carbon dioxide by pulmonary carbonic anhydrase. Science. 1978 Jan 27;199(4327):427–429. doi: 10.1126/science.413195. [DOI] [PubMed] [Google Scholar]
- Frankel H. M., Garcia E., Malik F., Weiss J. K., Weiss H. R. Effect of acetazolamide on cerebral blood flow and capillary patency. J Appl Physiol (1985) 1992 Nov;73(5):1756–1761. doi: 10.1152/jappl.1992.73.5.1756. [DOI] [PubMed] [Google Scholar]
- GIACOBINI E. A cytochemical study of the localization of carbonic anhydrase in the nervous system. J Neurochem. 1962 Mar-Apr;9:169–177. doi: 10.1111/j.1471-4159.1962.tb11859.x. [DOI] [PubMed] [Google Scholar]
- Hanson M. A., Nye P. C., Torrance R. W. The location of carbonic anhydrase in relation to the blood-brain barrier at the medullary chemoreceptors of the cat. J Physiol. 1981 Nov;320:113–125. doi: 10.1113/jphysiol.1981.sp013938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson M. A., Rao P. S., Torrance R. W. Carbon dioxide sensitivity of aortic chemoreceptors in the cat. Respir Physiol. 1979 Apr;36(3):301–309. doi: 10.1016/0034-5687(79)90043-4. [DOI] [PubMed] [Google Scholar]
- Holder L. B., Hayes S. L. Diffusion of sulfonamides in aqueous buffers and into red cells. Mol Pharmacol. 1965 Nov;1(3):266–279. [PubMed] [Google Scholar]
- Iturriaga R. Carotid body chemoreception: the importance of CO2-HCO3- and carbonic anhydrase. (review). Biol Res. 1993;26(3):319–329. [PubMed] [Google Scholar]
- Kjällquist A., Messeter K., Siesjö B. K. The in vivo CO2 buffer capacity of rat brain tissue under carbonic anhydrase inhibition. Acta Physiol Scand. 1970 Jan;78(1):94–102. doi: 10.1111/j.1748-1716.1970.tb04643.x. [DOI] [PubMed] [Google Scholar]
- LEE K. D., MATTENHEIMER H. THE BIOCHEMISTRY OF THE CAROTID BODY. Enzymol Biol Clin (Basel) 1964;10:199–216. doi: 10.1159/000458029. [DOI] [PubMed] [Google Scholar]
- Lahiri S., Mokashi A., Mulligan E., Nishino T. Comparison of aortic and carotid chemoreceptor responses to hypercapnia and hypoxia. J Appl Physiol Respir Environ Exerc Physiol. 1981 Jul;51(1):55–61. doi: 10.1152/jappl.1981.51.1.55. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Carbonic anhydrase: chemistry, physiology, and inhibition. Physiol Rev. 1967 Oct;47(4):595–781. doi: 10.1152/physrev.1967.47.4.595. [DOI] [PubMed] [Google Scholar]
- Maren T. H. Use of inhibitors in physiological studies of carbonic anhydrase. Am J Physiol. 1977 Apr;232(4):F291–F297. doi: 10.1152/ajprenal.1977.232.4.F291. [DOI] [PubMed] [Google Scholar]
- Olievier C. N., Berkenbosch A., Quanjer P. H. In vivo measurement of carbon dioxide tension with a miniature electrode. Pflugers Arch. 1978 Mar 20;373(3):269–272. doi: 10.1007/BF00580834. [DOI] [PubMed] [Google Scholar]
- Pontén U., Siesjö B. K. Gradients of CO2 tension in the brain. Acta Physiol Scand. 1966 Jun;67(2):129–140. doi: 10.1111/j.1748-1716.1966.tb03294.x. [DOI] [PubMed] [Google Scholar]
- ROTH L. J., SCHOOLAR J. C., BARLOW C. F. Sulfur-35 labeled acetazolamide in cat brain. J Pharmacol Exp Ther. 1959 Feb;125(2):128–136. [PubMed] [Google Scholar]
- Read D. J., Leigh J. Blood-brain tissue Pco2 relationships and ventilation during rebreathing. J Appl Physiol. 1967 Jul;23(1):53–70. doi: 10.1152/jappl.1967.23.1.53. [DOI] [PubMed] [Google Scholar]
- Ridderstråle Y., Hanson M. Histochemical study of the distribution of carbonic anhydrase in the cat brain. Acta Physiol Scand. 1985 Aug;124(4):557–564. doi: 10.1111/j.1748-1716.1985.tb00048.x. [DOI] [PubMed] [Google Scholar]
- Ringelstein E. B., Van Eyck S., Mertens I. Evaluation of cerebral vasomotor reactivity by various vasodilating stimuli: comparison of CO2 to acetazolamide. J Cereb Blood Flow Metab. 1992 Jan;12(1):162–168. doi: 10.1038/jcbfm.1992.20. [DOI] [PubMed] [Google Scholar]
- Swenson E. R. The respiratory aspects of carbonic anhydrase. Ann N Y Acad Sci. 1984;429:547–560. doi: 10.1111/j.1749-6632.1984.tb12386.x. [DOI] [PubMed] [Google Scholar]
- TRAVIS D. M., WILEY C., NECHAY B. R., MAREN T. H. SELECTIVE RENAL CARBONIC ANHYDRASE INHIBITION WITHOUT RESPIRATORY EFFECT: PHARMACOLOGY OF 2-BENZENESULFONAMIDO-1,3, 4-THIADIAZOLE-5-SULFONAMIDE (CL 11,366). J Pharmacol Exp Ther. 1964 Mar;143:383–394. [PubMed] [Google Scholar]
- Teppema L. J., Rochette F., Demedts M. Effects of acetazolamide on medullary extracellular pH and PCO2 and on ventilation in peripherally chemodenervated cats. Pflugers Arch. 1990 Feb;415(5):519–525. doi: 10.1007/BF02583501. [DOI] [PubMed] [Google Scholar]
- Teppema L. J., Vis A., Evers J. A., Folgering H. T. Dynamics of brain extracellular fluid pH and phrenic nerve activity in cats after end-tidal CO2 forcing. Respir Physiol. 1982 Dec;50(3):359–380. doi: 10.1016/0034-5687(82)90029-9. [DOI] [PubMed] [Google Scholar]
- Travis D. M., Wiley C., Maren T. H. Respiration during chronic inhibition of renal carbonic anhydrase: further observations on pharmacology of 2-benzenesulfonamido-1,3, 4-thiadiazole-5-sulfonamide (CL 11,366), acetazolamide and methazolamide. J Pharmacol Exp Ther. 1966 Mar;151(3):464–481. [PubMed] [Google Scholar]
- Vorstrup S., Henriksen L., Paulson O. B. Effect of acetazolamide on cerebral blood flow and cerebral metabolic rate for oxygen. J Clin Invest. 1984 Nov;74(5):1634–1639. doi: 10.1172/JCI111579. [DOI] [PMC free article] [PubMed] [Google Scholar]


