FIGURE 1.
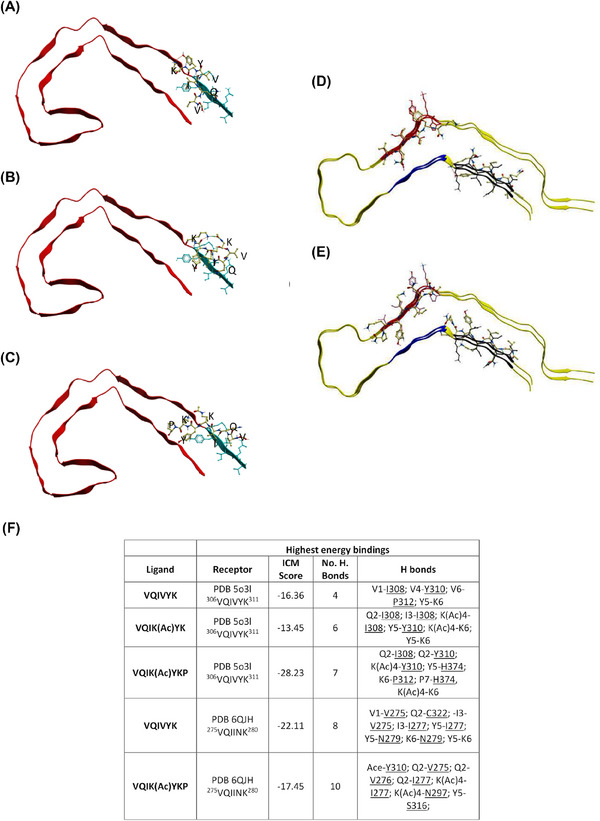
VQIK(Ac)YKP peptides dock to VQIVYK. Experimental peptides docked in parallel to the superior portion of PDB 5o3l 2N4R PHF (A–C) demonstrate preferential binding to 306VQIVYK311, whereas experimental peptides docked to PDB 6QJH 2N4R Tau snake filament (D–E) bind to both 306VQIVYK311 and 275VQIINK280. (A) Ac‐VQIVYK‐NH2; (B) AC‐VQIK(Ac)YK‐NH2; (C) Ac‐VQIK(Ac)YKP‐NH2, notice the peptide extending to interact with the parallel β‐sheet of 306VQIVYK311. (D) Ac‐VQIVYK‐NH2 binding in parallel to the filament; (E) Ac‐VQIK(Ac)YKP‐NH2 binding in anti‐parallel to the filament at 306VQIVYK311 position, and in parallel at the 275VQIINK280 position. (F) Summary table of the highest computationally calculated energy values describing the docked compounds to PDB‐5o3l and PDB‐5QJH (emphasis on VQIINK). Lower scores indicate more powerful interactions. ICM score of <–32 indicates a strong binding.
