FIGURE 4.
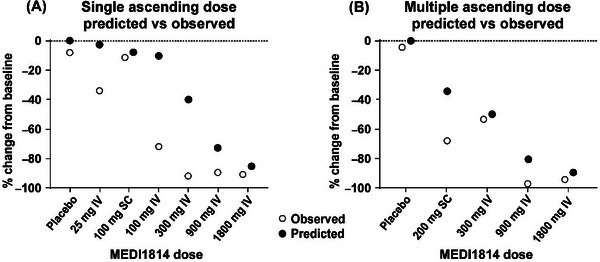
Comparison of observed and predicted pharmacodynamic responses to MEDI1814 after administration to patients with AD. Data show median % change from baseline in CSF free Aβ42 after administration of placebo and MEDI1814 single doses (25, 100, 300, 900, and 1800 mg IV or 100 mg SC in the single ascending dose study [A]) or repeat doses (300, 900, and 1800 mg IV or 200 mg SC, administered monthly in the multiple ascending dose study [B]). Relative to baseline, a dose‐dependent reduction of free Aβ42 in CSF and increase in total (bound and free) Aβ42 was observed at day 29 after single MEDI1814 doses (A). Near maximal levels of suppression were observed after the 900 and 1800 mg IV repeat doses (B). Aβ, amyloid beta; AD, Alzheimer's disease; CSF, cerebrospinal fluid; assay; IV, intravenous; SC, subcutaneous.
