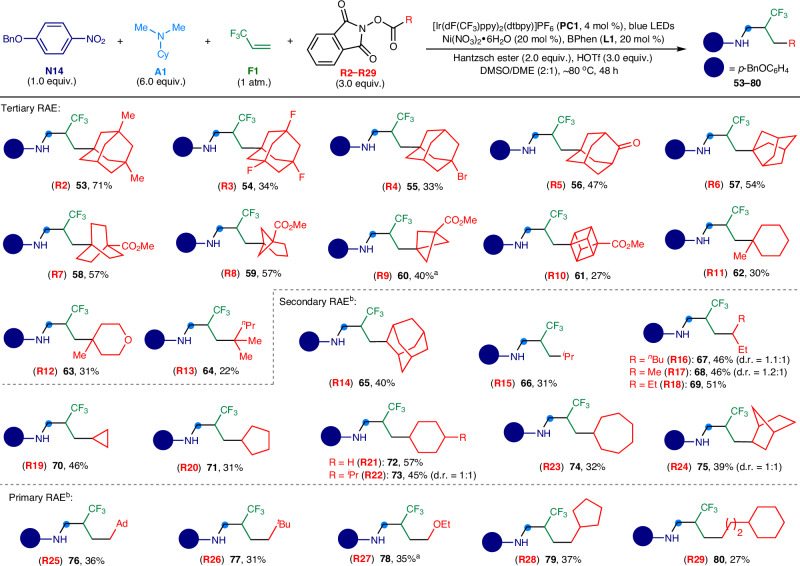
Fig. 4. Scope of redox active esters.
General reaction conditions: nitroarene (N14, 1.0 equiv., 0.15 mmol), tertiary alkylamine (A1, 6.0 equiv., 0.90 mmol), 3,3,3,-trifluoropropene (F1, 1 atm.), redox-active ester (R2 − R29, 3.0 equiv., 0.45 mmol), PC1 (4 mol %), Ni(NO3)2•6H2O (20 mol %), bathophenanthroline (BPhen, L1: 20 mol %), Hantzsch ester (2.0 equiv., 0.30 mmol), HOTf (3.0 equiv., 0.45 mmol), DMSO/DCE (v/v = 2:1, 6 mL), ~80 oC, blue LEDs (30 W, 456–460 nm), 48 h. aKessil LEDs (40 W, 456 nm) were used instead. bNi(bipy)Cl2 (20 mol %) were used instead. Ad, 1-adamantyl; Me, methyl; nPr, n-propyl; iPr, isopropyl, nBu, n-butyl; Et, ethyl; tBu, tert-butyl; bipy = 2,2′-bipyridine.